The planet we inhabit is surrounded by a vast expanse of air, known as the atmosphere. However, extraterrestrial beings visiting from other regions of our solar system do not share our fascination with Earth’s atmospheric makeup. Interestingly, even the most primitive life forms that first emerged on our planet did not find the prevailing air composition suitable for their survival. Nevertheless, the unique blend of nitrogen and oxygen that humans refer to as air has proven to be conducive to the flourishing of Earth’s diverse inhabitants.
The Presence of Air
The presence of air on our planet, as well as on other celestial bodies, predates the formation of the Earth itself. The composition of Earth’s current atmosphere has undergone a series of changes that began with the formation of our solar system.
The Early Atmosphere of Earth
The early atmosphere of Earth, much like the dust and rocks that contributed to the formation of our planet, originated during the early stages of the solar system. This initial atmosphere consisted of a thin layer composed primarily of hydrogen and helium, which was later stripped away by the tumultuous activity of the hot rocks that eventually coalesced to form Earth. This temporary atmosphere of hydrogen and helium originated from the remnants of the gaseous mass that eventually transformed into our sun.
Earth’s subsequent atmospheric composition
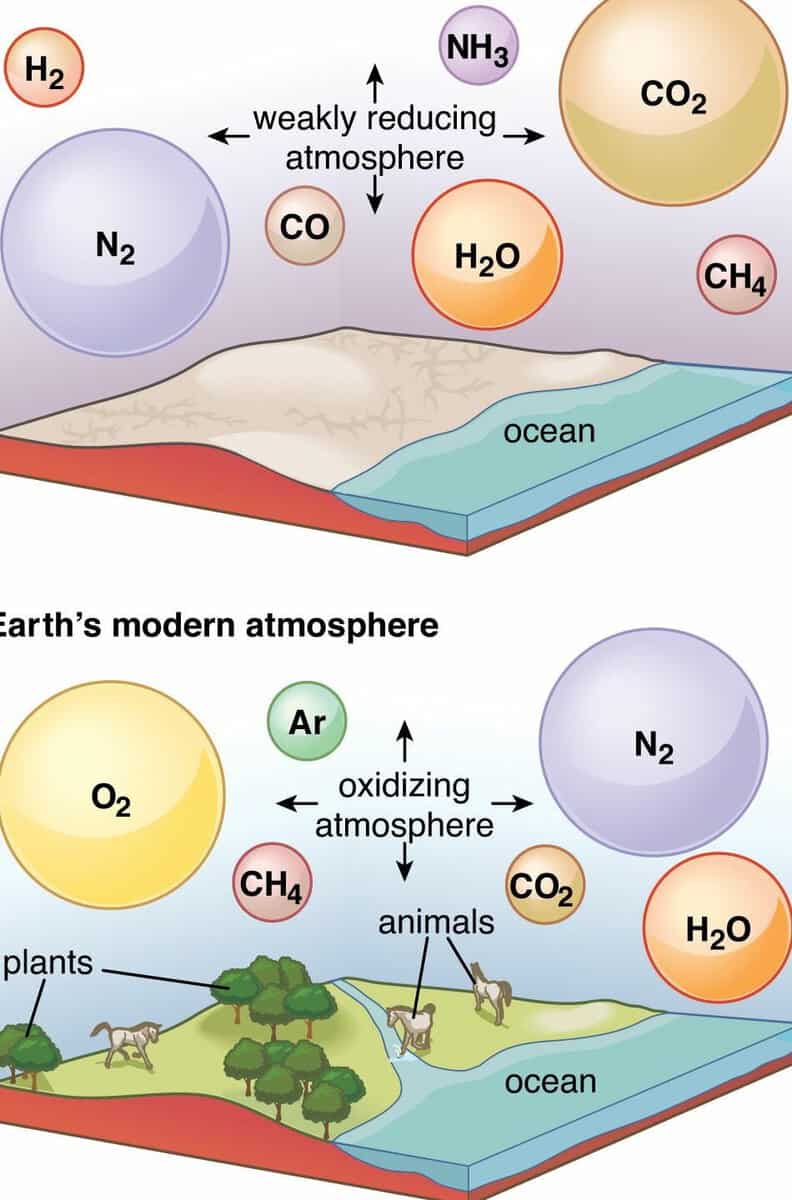
The gradual solidification of the once molten rock that eventually gave rise to Earth took an extensive period to complete. Volcanic activity persisted for millions of years, expelling gases from the planet’s interior. The primary gases emitted included carbon dioxide, water vapor, hydrogen sulfide, and ammonia. As time elapsed, these gases accumulated, giving rise to Earth’s subsequent atmospheric composition. Approximately 500 million years later, Earth had cooled sufficiently for water to amass, initiating a further cooling process that eventually culminated in the formation of Earth’s initial ocean.
Earth’s current atmosphere, the third in its history
The first identifiable fossils on Earth, which were microscopic bacteria, have been dated to around 3.8 billion years ago. Around 2.7 billion years ago, cyanobacteria began to populate the Earth’s oceans. These cyanobacteria played a crucial role in producing oxygen through the process of photosynthesis. As a result, the levels of atmospheric oxygen increased, while the levels of carbon dioxide decreased, as it was consumed by the photosynthetic cyanobacteria.
Simultaneously, the sunlight caused atmospheric ammonia to break down into nitrogen and hydrogen. Most of the lighter hydrogen gas rose and eventually escaped into space. However, nitrogen gradually accumulated in the atmosphere over time.
Around 2.4 billion years ago, there was a significant rise in nitrogen and oxygen levels in the atmosphere, resulting in a shift from an earlier reducing atmosphere to the oxidizing atmosphere that exists today. The present-day atmosphere is primarily made up of 78 percent nitrogen, 21 percent oxygen, 0.9 percent argon, 0.03 percent carbon dioxide, and trace amounts of other gases. This composition is relatively stable due to the balance between photosynthesis by plants and bacteria and animal respiration.
Existence in a vast expanse of air
The majority of Earth’s weather and biodiversity takes place in the troposphere, which is the closest layer of the atmosphere to the Earth’s surface. At sea level, the atmospheric pressure amounts to 14.70 pounds per square inch (psi). This pressure is the result of the weight of the entire column of air above each individual square inch of surface area. So where does the air inside a car come from? As cars are not hermetically sealed containers, the pressure from the air surrounding and above the car pushes air into the car.
However, where does the air inside an airplane come from? Airplanes are more airtight compared to cars, but they are not completely impermeable. The pressure from the air surrounding and above the airplane fills the airplane with air. Unfortunately, modern airplanes fly at altitudes of 30,000 feet or higher, where the air is too sparse for humans to breathe.
To maintain higher air pressures at higher altitudes, it is necessary to enhance the strength of the aircraft’s body structure. The outer shell needs to be stronger when there is a greater difference between the internal and external air pressures. In airplane cockpits, a pressure equivalent to 7,000 feet above sea level, approximately 11 pounds per square inch, is often utilized. This pressure level ensures comfort for most individuals while simultaneously reducing the overall weight of the aircraft.
Air can be found almost everywhere
So where does the air in water that is boiling come from? In simple terms, it’s air that has been dissolved in the water. The amount of air that can dissolve in water depends on the temperature and pressure. As the temperature rises, the amount of air that can dissolve in water decreases. When the water reaches its boiling point of 212 °F (100 °C), the dissolved air escapes from the water. Because air is less dense than water, air bubbles rise to the surface.
On the other hand, the amount of air that can dissolve in water increases with higher pressure. The boiling point of water decreases as the altitude increases because the air pressure decreases. By using a lid, the pressure on the surface of the water increases, raising the boiling point. Adjustments to recipes are needed when cooking at higher altitudes due to the effect of lower pressure on the boiling point.
What is the source of collagen?
Collagen, which is an essential protein found in cartilage, is derived from deceased animals and is utilized in the form of gelatin for various purposes including food consumption, medical applications, and cosmetic procedures.
What is the origin of iron or how is it produced?

On Earth, iron (Fe) is derived from iron ore, which consists of iron and various amounts of rock. Iron is the main component in the manufacturing process of steel. The origin of the iron element can be traced back to supernovae, which are the explosive demise of distant stars.

Nylon, a synthetic fiber that can be used as a substitute for silk, was invented in 1934 by Wallace Carothers, an organic chemist at EI du Pont de Nemours. This versatile material is now utilized in the production of clothing, tires, rope, and various other everyday items. Nylon is considered one of the earliest man-made fibers.
The Earth’s air envelope, known as the atmosphere, derives its name from the Greek words atmos (meaning vapor) and sphaira (meaning balloon). Unlike other layers of the Earth, the atmosphere does not have a distinct upper boundary. The majority of its mass, approximately 99.5%, is concentrated in the lower 80 km.
The release of gases during volcanic eruptions played a significant role in the formation of the atmosphere. Over time, the presence of oceans and the emergence of the biosphere further influenced its development.
Composition of the atmosphere
The atmosphere is composed of several distinct layers, each with its own characteristics such as temperature and density. The first and lowest layer is the troposphere, which is heated by the Earth’s surface and the Sun. The warmest part of the troposphere is closest to the Earth, and as you move higher, the temperature decreases. This temperature decrease is known as the vertical temperature gradient and averages 0.6°C for every 100 meters. The troposphere has varying thickness, with the equator having a thickness of 17 km and the polar latitudes having a thickness of 8-9 km. Weather phenomena like cloud formation and precipitation occur in this layer.
Above the troposphere is the stratosphere, separated by the tropopause. In the stratosphere, the air is less dense and there is no cloud formation due to the lack of water vapor. The temperature continues to decrease with altitude, but above 25 km it starts to increase due to the absorption of solar radiation by the ozone layer. This layer extends up to 50-55 km.
Beyond the stratosphere is the mesosphere, where the air becomes even more rarefied and the temperature starts to rise. The next layer is the thermosphere, where complex chemical reactions make the atmosphere electrically conductive. This layer, along with the mesosphere, is known as the ionosphere and is responsible for phenomena like the aurora borealis. The mesosphere and thermosphere extend up to 80-85 km.
The outermost layer of the atmosphere is the exosphere, where gas particles are extremely rare and the temperature can reach +2000°C. The gas composition of the atmosphere has been studied extensively, with oxygen and nitrogen being the main components. However, there are also other gases present in the air. Overall, the atmosphere is a mixture of gases that vary near the Earth’s surface.
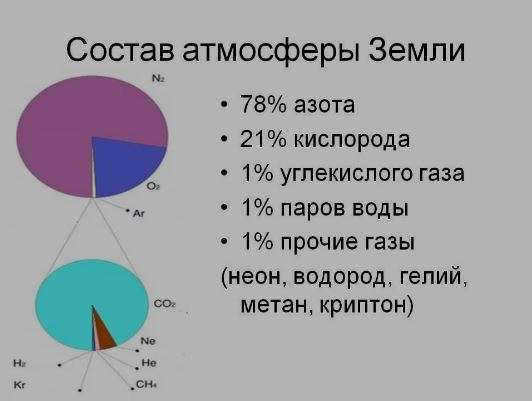
- Nitrogen accounts for 78% of the Earth’s atmosphere.
- Oxygen makes up 21% of the atmosphere.
- Inert gases, such as argon and helium, comprise 0.94% of the atmosphere.
- Carbon dioxide, a greenhouse gas, constitutes 0.03% of the atmosphere.
- Water vapor and impurities also make up 0.03% of the atmosphere.
The Significance of the Atmosphere in Nature and Human Life
- The presence of the gaseous envelope surrounding the Earth helps regulate temperature, preventing excessive heating during the day and excessive cooling at night, unlike the Moon’s surface, which lacks an atmosphere.
- The atmosphere acts as a shield, protecting the Earth from meteorites, with most of them burning up before reaching the planet’s surface.
- The ozone layer, or ozonosphere, safeguards humanity from excessive ultraviolet radiation, which can be harmful to living organisms.
- Oxygen, an essential component of the atmosphere, is vital for all living organisms to breathe and sustain life.

Exploring the Atmosphere
For centuries, humanity has been fascinated by the world above us, and it was only a few hundred years ago that the first tools were created to study the atmosphere. The thermometer, barometer, and weather vane were invented, marking the beginning of our understanding of the Earth’s gas envelope. Today, the World Meteorological Organization (WMO) leads the way in studying the atmosphere, with participation from numerous countries including Russia. They have developed a program that collects and processes data using the latest technology. A network of ground-based meteorological stations, equipped with a variety of instruments, has been established to monitor the condition of the atmosphere.
Temperature is typically measured using thermometers, and in Europe it is commonly measured in degrees “Celsius”. This particular unit of measurement is based on the physical properties of water: it freezes and becomes a solid at zero degrees, and it turns into a gas at 100 degrees. To measure the amount of precipitation, a precipitation gauge is used. This gauge is a container with markings on its walls that indicate the amount of precipitation. The velocity of air currents is measured using a wind gauge, also known as an anemometer. To determine the wind direction, a weather vane is usually installed next to the wind gauge. In areas where strong winds can be hazardous, such as airfields and bridges, windsocks are installed. These windsocks are large cone-shaped bags made of striped cloth and are open on both sides. Atmospheric pressure is measured using a barometer.

There are numerous little-known facts regarding the atmosphere that are rather astonishing.
Why does the atmosphere appear blue? It’s actually purple!
Every child in their initial drawings depicts the sky as blue. It may seem that way, simply by looking out the window on a clear day, one can be convinced of this. However, in reality, we perceive the atmosphere as blue due to the characteristics of vision and the optical laws of physics. If it were not for these factors, the atmosphere would appear purple.
As sunlight passes through gas and water vapor, it becomes scattered. Shorter wavelengths, including the violet portion of the spectrum, are absorbed more intensely. Nevertheless, human eyes are more sensitive to the color blue, meaning we perceive it more effectively than violet. Consequently, when we gaze at the sky, it appears blue to us.
The oxygen content in our atmosphere is less than 21% by volume. The majority of the atmosphere consists of nitrogen, which makes up approximately 78%, along with oxygen. The remaining gases make up only 1%. It is not widely known that the Earth does not have the highest oxygen content in its atmosphere. For instance, Mercury has approximately 42% of this gas. However, it is not breathable due to the planet’s highly rarefied atmosphere.

The atmosphere on Earth was formed thanks to cyanobacteria that lived in water.
In terms of atmospheric density, Earth ranks third among the celestial bodies in the solar system. Titan and Venus have denser atmospheres, with Venus having the highest density. The density on Venus is so high that it gradually becomes liquid near the surface and transitions smoothly into an ocean.
Interestingly, oxygen did not appear in such large quantities on Earth immediately. Scientists believe that the saturation of the atmosphere with oxygen is linked to the slowing down of Earth’s rotation caused by the presence of the Moon.
Subscribe to our exclusive Pulse Mail.ru newsletter, where you will discover a plethora of captivating and enriching content.
The essential role of greenhouse gases in the atmosphere
We are all aware of the excessive harm caused by greenhouse gases to our atmosphere, as they contribute to the alarming global climate warming phenomenon. Various scientific experts propose diverse strategies to mitigate the presence of these gases in the atmosphere. Some of these ideas may appear extraordinary. For instance, I recently shared with you the remarkable concept of scientists “resurrecting” the mammoth, with the aim of rejuvenating the Arctic pastures.
Nevertheless, the absence of greenhouse gases on our planet would result in an uncomfortable environment. The truth is that these gases play a crucial role in absorbing solar energy and ensuring its uniform distribution across Earth’s surface. Without them, the majority of the planet would experience extreme cold, while the equatorial region would be subjected to unbearable heat. Hence, the atmosphere possesses an optimal combination of gases that facilitates a comfortable existence, and any alteration in its composition could lead to severe repercussions.

The mark left by an aircraft in the atmosphere is, in reality, frozen water particles
What is the origin of airplane trails in the sky?
Have you ever pondered why a bright “ski line” appears in the sky after a jet plane, adding a touch of beauty to the blue backdrop? We have all observed it linger for a while before gradually dispersing. What we are actually witnessing up above is frozen water. It forms when the cold atmosphere intersects with the moist and warm exhaust from an aircraft. The resulting moisture instantaneously crystallizes into tiny ice particles.
If the trail is faint and dissipates quickly, it suggests that the aircraft is flying at a low humidity altitude. Conversely, if the trail is distinct and lingers for an extended period, it indicates high humidity in the air. This implies that the weather may soon deteriorate, with the possibility of a thunderstorm.
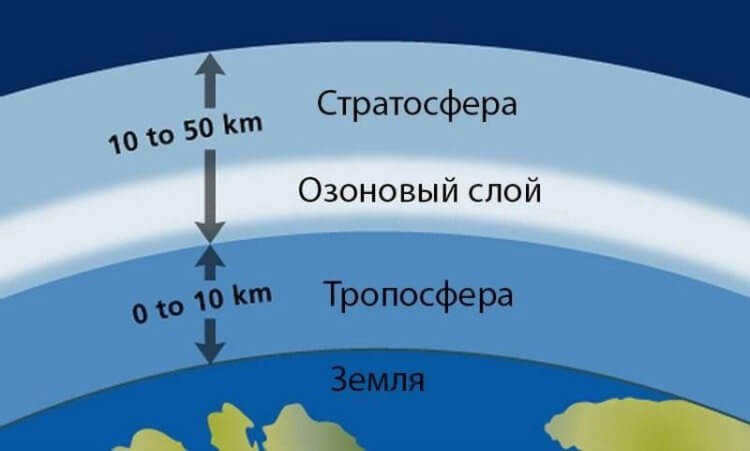
The troposphere is not as warm as the ozone layer.
The stratosphere is cooler than the troposphere.
Contrary to popular belief, the temperature in the atmosphere doesn’t decrease with altitude. In fact, the stratosphere, which is located higher up, is actually cooler than the troposphere. This is because the stratosphere contains the ozone layer, which plays a vital role in shielding the Earth’s inhabitants from harmful ultraviolet radiation.
The stratosphere warms up because it absorbs the heat from the sun. In the article about the ozone hole, we discussed the process of ozone formation. Finally, it is worth mentioning that the stratosphere is crucial as it serves as the airspace for jets and weather probes.

The Earth’s atmosphere is the gas envelope that surrounds the planet. It covers the hydrosphere and a portion of the Earth’s crust, while the outer surface borders near-Earth space. The atmosphere plays a significant role in shaping the weather conditions on the planet’s surface.
Even at an altitude of five kilometers above sea level, an untrained individual will experience oxygen deprivation and a decrease in performance. At 9 km above sea level, humans are unable to breathe, despite the presence of oxygen in the atmosphere up to approximately 115 km.
The atmosphere serves as the primary supplier of oxygen for human respiration. Nevertheless, as we ascend in altitude, the overall atmospheric pressure decreases, resulting in a decrease in partial oxygen pressure.

The atmosphere plays a crucial role in our respiratory system. Inside our lungs, we have about three liters of alveolar air. Under normal atmospheric pressure, the partial pressure of oxygen in this air is 11 mmHg, while the carbon dioxide pressure is 40 mmHg. Additionally, there is a water vapor pressure of 47 mmHg. However, as we ascend to higher altitudes, the oxygen pressure decreases while the water and carbon dioxide vapor pressures remain constant at a total of approximately 87 mmHg. When the ambient air pressure matches this value, the flow of oxygen into our lungs stops.
As a result of the decrease in atmospheric pressure at an elevation of 20 kilometers, the water and fluids within the human body will reach boiling point. In the absence of a pressurized cabin, a person would succumb to immediate death at this altitude. Thus, from a physiological standpoint, the threshold for “space” begins at 20 km above sea level.
The atmosphere plays a crucial role in the sustenance of life on Earth. The dense layers of the atmosphere, namely the troposphere and stratosphere, provide protection against harmful radiation. In the vacuum of space, where the air is thin, ionizing radiation becomes active at altitudes exceeding 36 km. Furthermore, ultraviolet radiation becomes prevalent above 40 kilometers.
As you ascend above the Earth’s surface to an altitude of more than 90-100 km, there will be a gradual weakening and eventual disappearance of the familiar phenomena observed in the lower atmospheric layer:
- Sound propagation ceases.
- There is no aerodynamic force and drag.
- Heat is not transferred through convection, among other things.
Arrangement of the Earth’s Atmosphere Layers
The Earth’s atmosphere is organized into distinct layers, which are arranged in the following order from the planet’s surface:
There are no clear boundaries between each layer, and their heights are influenced by factors such as latitude and the changing seasons. This layered structure developed due to variations in temperature at different altitudes. It is thanks to the atmosphere that we are able to observe the phenomenon of twinkling stars.
Overview of the Earth’s Atmosphere Layers:
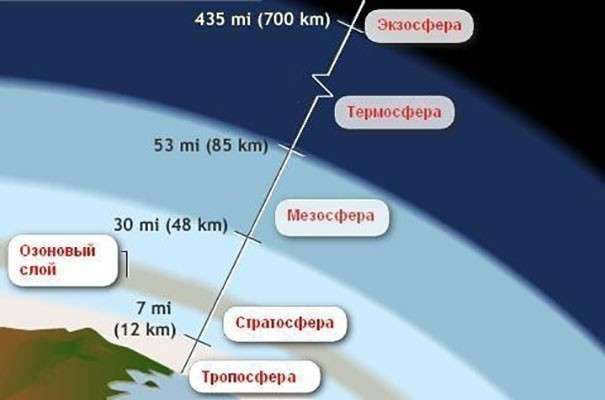
What is the composition of Earth’s atmosphere?
Earth’s atmosphere is composed of different atmospheric layers, each having its own temperature, density, and composition. The total thickness of the atmosphere is approximately 1.5-2.0 thousand kilometers. The current composition of Earth’s atmosphere consists of a mixture of gases with various impurities.
The structure of Earth’s atmosphere starts with the troposphere, which is the lower part of the atmosphere extending to a height of about 10-15 km. This layer contains the majority of atmospheric air. One notable characteristic of the troposphere is a temperature decrease of 0.6˚C for every 100 meters of elevation. The troposphere also holds most of the atmospheric water vapor, making it the primary location for cloud formation.

The troposphere’s height undergoes daily fluctuations. Moreover, its average measurement varies based on latitude and season. The average altitude of the troposphere above the polar regions is 9 km, while above the equator, it reaches approximately 17 km. The average yearly air temperature above the equator hovers around +26 ˚C, whereas above the North Pole, it drops to -23 ˚C. At the upper boundary of the troposphere, the average annual temperature above the equator is about -70 ˚C, while above the North Pole, it ranges from -45 ˚C in the summer to -65 ˚C in the winter. As a result, the temperature decreases with increasing altitude. The sun’s rays pass through the troposphere unhindered, warming the Earth’s surface. However, the heat emitted by the sun gets trapped by carbon dioxide, methane, and water vapor.
Located above the troposphere is the stratosphere, which spans a height of 50-55 km. One unique characteristic of this layer is the temperature increase as you go higher. Acting as a transition between the troposphere and the stratosphere is a layer known as the tropopause.
Starting at around 25 kilometers in height, the temperature in the stratospheric layer begins to rise, reaching its peak at 50 km with temperatures ranging from +10 to +30 ˚C.

The stratosphere contains a minimal amount of water vapor. Occasionally, at approximately 25 km above the Earth’s surface, there are thin clouds known as “pearlescent” clouds. These clouds are not visible during the day but emit a glow at night, caused by the sun’s illumination below the horizon. Pearlescent clouds consist of supercooled water droplets. The majority of the stratosphere is composed of ozone.
The mesosphere layer extends to a height of around 80 kilometers. As you ascend, the temperature drops and can reach several tens of degrees below zero at its uppermost boundary. Within the mesosphere, one can also observe clouds, which are believed to be composed of ice crystals. These clouds are commonly referred to as “silvery” clouds. The mesosphere is notable for having the lowest temperatures in the atmosphere, ranging from -2 to -138 degrees Celsius.

The thermosphere got its name because of its elevated temperatures. This layer of the atmosphere is made up of:
The ionosphere is known for its thin air, with each centimeter at a height of 300 km containing 1 billion atoms and molecules, and at a height of 600 km containing over 100 million.

The ionosphere is distinguished by the presence of highly ionized air. These ions consist of oxygen atoms with a charge, nitrogen molecules with a charge, and free electrons.
At an altitude of 800-1000 km, the exospheric layer begins. Gas particles, especially light ones, move here at high speeds, surpassing the gravitational force. Due to their rapid movement, these particles escape from the atmosphere into outer space and disperse. That is why the exosphere is often referred to as a scattering sphere. The particles that escape into space are mostly hydrogen atoms, which form the uppermost layers of the exosphere. It is thanks to these particles and solar wind particles that we are able to witness the phenomenon of the aurora borealis.

Thanks to the use of satellites and geophysical rockets, scientists have been able to confirm the existence of the Earth’s radiation belt in the upper atmosphere. This belt is composed of electrically charged particles, specifically electrons and protons.
Watch this video to learn more about the composition of the Earth’s atmosphere.
How was the Earth’s atmosphere formed?
Scientists have long been studying the origin and composition of the Earth’s atmosphere. The prevailing theory suggests that the atmosphere initially consisted of three distinct compositions.
- Initially, approximately 4 billion years ago, the atmosphere was comprised of light gases obtained from outer space.
- During the second stage, around 3 billion years ago, the atmosphere underwent active volcanic activity, resulting in the presence of additional gases such as ammonia, carbon dioxide, and water vapor.
- The subsequent formation of the atmosphere was influenced by the escape of light gases (helium and hydrogen) and chemical reactions that occurred due to ultraviolet radiation, lightning discharges, and other factors.
When living organisms first appeared on the planet, the composition of the Earth’s original atmosphere underwent a radical transformation as a result of the release of oxygen and the absorption of carbon dioxide through photosynthesis. The amount of oxygen in the atmosphere increased, leading to the development of the modern atmosphere with its oxidizing properties. This, in turn, caused significant changes in the processes taking place in the atmosphere, biosphere, and lithosphere. In scientific terms, this period is referred to as the “oxygen catastrophe.”
Currently, the Earth’s atmosphere consists of a variety of gases and various pollutants. The levels of atmospheric gases remain relatively stable, with the exception of water vapor and carbon dioxide.
Here is the breakdown of the Earth’s atmosphere composition in percentage:
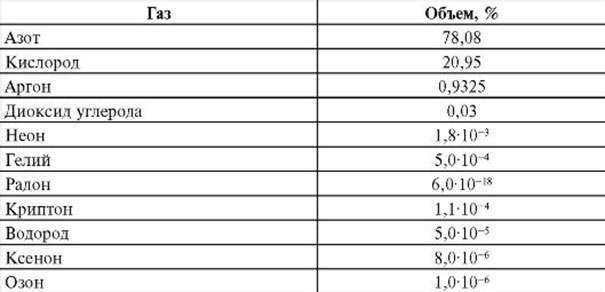
Air primarily consists of nitrogen, which makes up around 78% of the Earth’s atmosphere. This nitrogen is formed through the oxidation of the hydrogen-ammonia atmosphere by oxygen molecules derived from photosynthesis on the Earth’s surface. Oxygen is the second most abundant gas, making up nearly 21% of the atmosphere, while radon is the least abundant gas.
If the Earth had no atmosphere, what do you think it would be like? Leave your thoughts in the comments.
Check out this video on the different layers of Earth’s atmosphere: Universe Edge of Space Earth troposphere, stratosphere, mesosphere, thermosphere, exosphere


Let’s comprehend the whereabouts of these entities, shall we? As you are aware, our planet is encompassed by a shell composed primarily of gases. The Earth’s atmosphere is a representation of this very shell. It is important to note that it pertains to one of the geospheres, so to speak.
The planet’s atmosphere is an extension of it, as the gaseous mass moves along with the Earth. And it only gradually and seamlessly transitions into the cosmos.
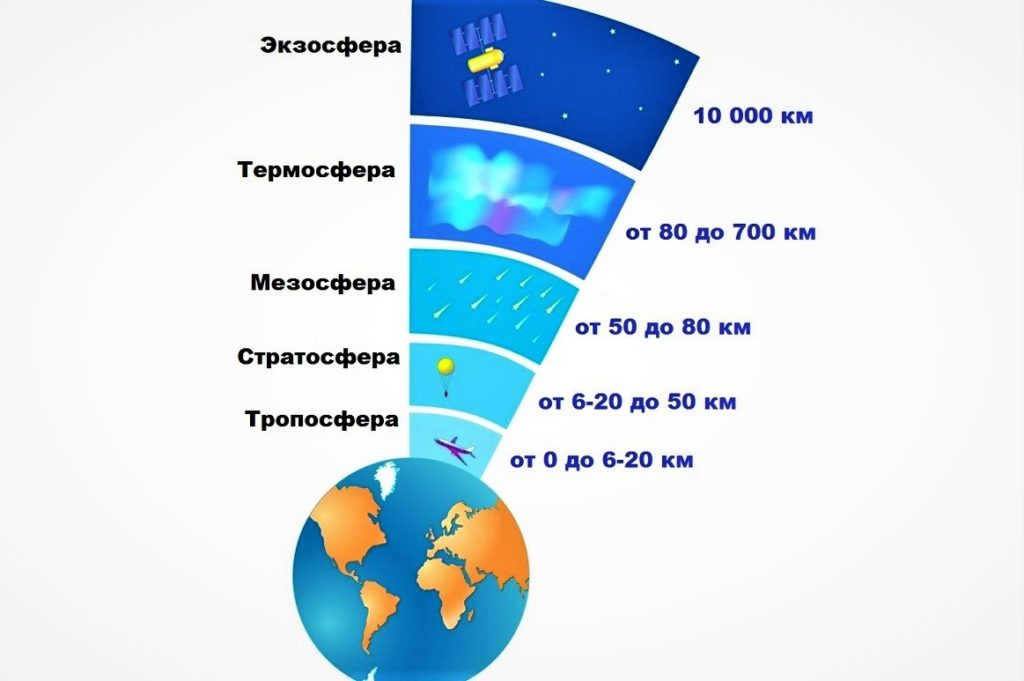
The Composition of the Earth’s Atmosphere
The Earth’s atmosphere is formed through a combination of two factors:
- The impact of celestial objects on the Earth’s surface, causing the vaporization of their constituent substances;
- The release of gases from the Earth’s mantle during volcanic eruptions.
Additionally, the presence of water, plants, and animals on the planet has played a crucial role in shaping the atmosphere. These factors have led to the development of the biosphere and subsequent changes in the composition of the atmosphere.
Structure of the Earth’s Atmosphere
It is evident that the gaseous envelope enveloping our planet is far more than a mere veneer of water and air. It can be likened to a protective blanket, shielding us from the hazards of outer space. Currently, scientists have delineated distinct layers comprising the Earth’s atmosphere. Let us delve into each layer and explore their unique characteristics.
Troposphere
The troposphere is the primary and lowermost layer of the Earth’s atmosphere. It contains more than 80% of the total mass of air and approximately 90% of the water vapor present in the entire atmosphere. The upper boundary of this layer varies between 8 and 18 kilometers, depending on the geographic latitude.
The troposphere is characterized by convection and turbulence. It is where clouds form and where cyclones and anticyclones are created. Scientists have also observed a unique characteristic of this atmospheric layer: the air temperature decreases with increasing altitude.
Incidentally, the lower region of the troposphere functions as a boundary layer, spanning approximately 1-2 kilometers. Interestingly enough, it exhibits a strong connection to the Earth’s surface, as the properties and condition of our planet’s sphere have a significant impact on the encompassing shell.

Tropopause
The tropopause is the boundary where the troposphere transitions into the stratosphere. It is characterized by a gradual change from one layer to the other. It is worth noting that there is a halt in the decrease of air temperature as altitude increases.
This region of the atmosphere is situated at an elevation of 11 to 50 km. It is of utmost significance as it is where the ozone layer is located, which is renowned for safeguarding us against ultraviolet radiation.
The stratosphere constitutes approximately 20% of the Earth’s overall shell mass.
An distinguishing characteristic is that the lower section (11-25 km) experiences a slight temperature fluctuation, while the upper section (25-40 km) undergoes a significant temperature rise. Incidentally, the upper section is referred to as the inversion region.

Stratopause
It is worth noting that at the altitude of 40 km, the temperature remains at 00C and continues until 55 km. This region is known as the stratopause. By the way, it marks the boundary of the stratosphere and the transition to the mesosphere.
The mesosphere
The mesosphere begins at an altitude of 50 kilometers and extends up to 80-90 kilometers. It is in this region that the temperature decreases as altitude increases, according to scientific research.
The mesosphere is responsible for radiant heat exchange, as well as hosting intricate photochemical processes that result in the Earth’s atmospheric glow.
Comprising only 0.3% of the total mass, the mesosphere is a relatively small component of our atmosphere.

Mesopause
The mesopause is the region between the mesosphere and the thermosphere. It is important to mention that the temperature here is extremely low (around -90 ° C).
Karman line
Essentially, the Karman line is the pinnacle point above the Earth’s surface. Furthermore, it serves as the demarcation between the Earth’s atmosphere and outer space. It has been determined that the Karman line is situated at an elevation of 100 km above sea level.

The Atmosphere of Earth and Its Thermosphere
The Earth’s atmosphere and its thermosphere can be considered as the highest limit of the planet’s air zone (around 800 km). However, the temperature varies throughout this region. For instance, there is an increase in temperature up to 1500 K up to 200-300 km, and it remains at that level afterwards.
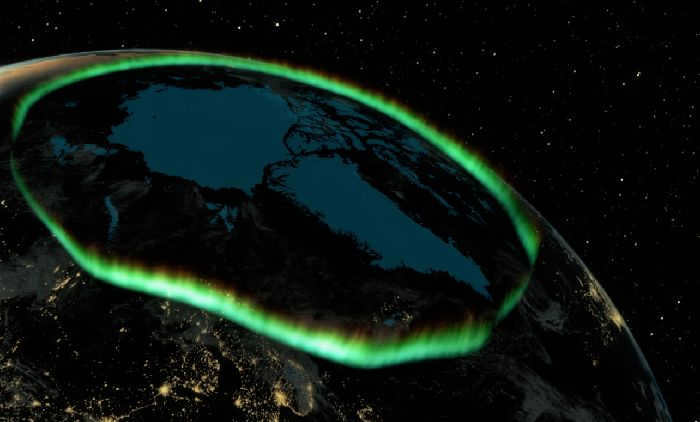
The presence of polar lights in this region can most likely be attributed to air ionization, which occurs as a result of the influence of solar and cosmic radiation. It is worth noting that the ionosphere, which is the main region for ionization, is located in this area.
Furthermore, at altitudes above 300 km, there is a significant concentration of atomic oxygen.
The upper boundary of the thermosphere can vary in size, primarily due to solar activity. During periods of low solar activity, the boundary decreases, and vice versa.
Out of the Earth’s total atmospheric mass, the thermosphere only accounts for slightly less than 0.05%.
Thermopause
The thermopause is the uppermost layer of the thermosphere. It experiences a slight absorption of solar radiation, while maintaining a constant temperature.
The exosphere
Also known as the exosphere, it is the outermost region of the thermosphere. It is characterized by an incredibly low density of gas particles, causing them to escape into space.
Around 2000-3000 km, the exosphere gradually transitions into the interplanetary space, earning the nickname of the near-space vacuum. This area is primarily occupied by rare gas particles, predominantly hydrogen atoms.

What are the other components of the Earth’s atmosphere?
In addition to the territorial aerial land layers, there are two other layers known as the ionosphere and the neutrosphere. These layers are distinguished by their electrical properties. As mentioned before, the ionosphere is mainly located in the thermosphere and is associated with air ionization.
However, not everyone understands what the neutrosphere is. Simply put, it is the lower part of the atmospheric layer where uncharged air particles of the Earth are predominant.

Furthermore, within the atmospheric envelope surrounding us, scientists have identified two distinct regions:
1) The heterosphere – this is where gravitational forces act upon the gases, resulting in a slight mixing of the gases. As a result, the composition of the heterosphere is not constant.
2) The homosphere – located beneath the heterosphere, this region is characterized by a more thorough mixing of gases. Therefore, the composition of the homosphere is uniform.
In addition, there exists a boundary between these regions known as the turbopause. It extends up to an altitude of 120 kilometers.