An atmosphere is a layer of gas that surrounds a celestial body, such as Earth, and is held in place by gravity. It serves several crucial functions, including shielding the planet from harmful ultraviolet radiation emitted by the sun, regulating temperature, and acting as a barrier against meteorites. The unique composition and characteristics of Earth’s atmosphere are what make it suitable for supporting life. However, many individuals are curious about the origins of our atmosphere and how it came to be.
With that in mind, we have decided to dedicate this article to providing you with insights into the formation of Earth’s atmosphere, its timeline, and the processes that contributed to its development.
Origin of the atmosphere
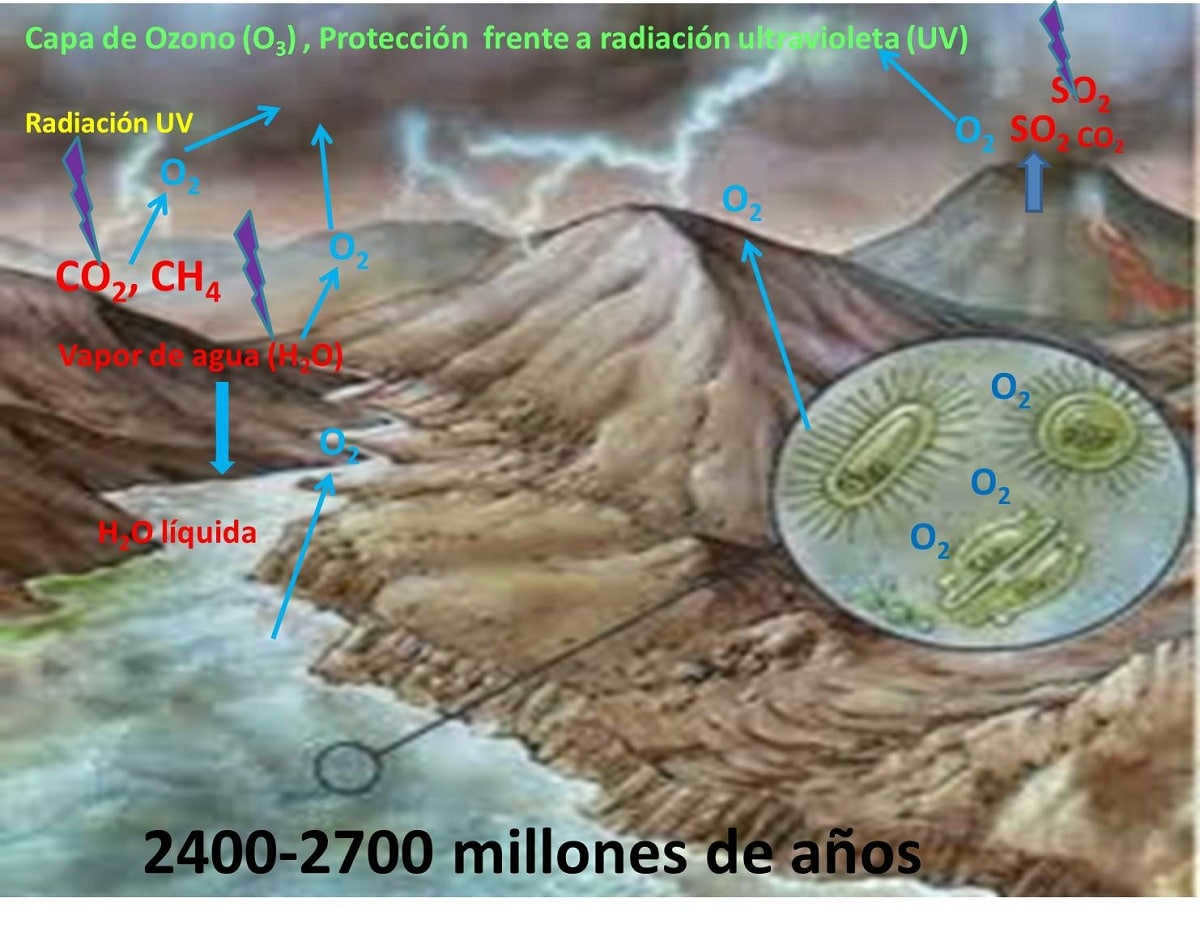
The formation of the Earth’s atmosphere is believed to have occurred through a process known as outgassing. During the early stages of the Earth’s formation, volcanic activity released gases such as water vapor, carbon dioxide, nitrogen, and methane. These gases gradually accumulated and formed the primitive atmosphere.
Over time, the Earth’s primitive atmosphere underwent significant changes. The development of photosynthetic organisms, such as cyanobacteria, led to the release of oxygen through the process of photosynthesis. This oxygen gradually built up in the atmosphere, leading to the development of an oxygen-rich atmosphere.
Today, the Earth’s atmosphere is composed primarily of nitrogen (about 78%) and oxygen (about 21%), with trace amounts of other gases such as carbon dioxide, methane, and ozone. The atmosphere plays a crucial role in supporting life on Earth, providing oxygen for respiration and regulating the planet’s temperature through the greenhouse effect.
The Earth is surrounded by a layer of gas known as the atmosphere, which exists thanks to the gravitational force of our planet. About 4.6 billion years ago, when the Earth was formed, the process of atmospheric formation began. Over the course of the first 500 million years, the atmosphere went through a series of changes. As the Earth’s interior continued to evolve, it released vapors and gases, causing the atmosphere to become unusually dense. The primary components of the early atmosphere included hydrogen (H2), water vapor, methane (CH4), helium (He), and carbon oxides. This initial atmosphere was not fully developed 200 million years ago, as the Earth was still too hot, leading to the escape of lighter gases.
The gravity of the Earth is slightly less than it is now, preventing the Earth from retaining molecules in the atmosphere; the magnetosphere has not yet formed and the solar wind directly impacts the surface. As a result, a significant portion of the original atmosphere has been lost to space.
Due to its temperature, size, and average mass, our planet is unable to retain light gases like hydrogen and helium, which escape into space and are carried away by the solar wind. Even with Earth’s current mass, it cannot support gases like helium and hydrogen, unlike larger planets such as Jupiter and Saturn, which have atmospheres rich in gas. The rocks that formed our planet continually released new gases and water vapor over a significant period of time until approximately 4 billion years ago when the atmosphere became predominantly composed of carbon molecules. These include carbon dioxide (CO2), carbon monoxide (CO), water (H2O), nitrogen (N2), and hydrogen (H).
The development of the hydrosphere began around 4 billion years ago due to the presence of certain compounds and a decrease in Earth’s temperature below 100°C.
Over time, water vapor condensed and accumulated, leading to the start of the deposition process. The existence of water facilitated the dissolution of gases like sulfur dioxide, hydrochloric acid, and carbon dioxide. These gases reacted with the lithosphere, resulting in the formation of acids and creating a reducing atmosphere. Methane and ammonia were also present.
In the 1950s, American researcher Stanley Miller conducted a well-known experiment using electrical discharges to generate a mixture of amino acids in this environment. This experiment showcased the ability of external energy to produce organic compounds.
With this aim in mind, he plans to replicate the original atmospheric circumstances that might have sparked the emergence of life. It is widely acknowledged that there are three essential prerequisites for life as we comprehend it: a stable atmosphere abundant in substances like oxygen and hydrogen, a continuous supply of external energy, and liquid water. As we have observed, the living conditions are nearly in place. However, the absence of free oxygen could delay the advent of life by millions of years. Rocks containing small amounts of elements like uranium and iron indicate an oxygen-free atmosphere. Consequently, these elements are absent in rocks from the middle Precambrian period or at least 3 billion years later.
The significance of oxygen

The formation of oxygen is the most crucial atmospheric process for organisms like us. Oxygen is not produced by direct chemical processes or geologic processes such as volcanic activity. Therefore, the conditions for the formation of proteins in the ocean and the process of condensation and synthesis of amino acids, nucleic acids, which carry the genetic code, in 1,500 million years, are the hydrosphere, a stable atmosphere, and energy from the sun. Later, single-celled anaerobic organisms appear in the ocean. Just a billion years ago, cyanobacteria, aquatic organisms, began using solar energy to break down molecules.
Genesis and Composition of the Earth’s Atmosphere
At this particular moment, certain oxygen molecules in the atmosphere assimilate the energy of the sun’s ultraviolet rays and divide to create individual oxygen atoms. These atoms merge with the remaining oxygen to generate ozone (O3) molecules, which absorb ultraviolet radiation from the sun. Throughout a span of 4 billion years, the quantity of ozone was insufficient to obstruct ultraviolet penetration, which would have hindered the existence of life beyond the oceans. About 600 million years back, with the assistance of marine life, the Earth’s atmosphere attained elevated ozone levels capable of absorbing detrimental ultraviolet light, resulting in the development of life on the continents. At this juncture, oxygen levels are approximately 10% of their current value. This is why life was previously confined to the ocean. Nonetheless, the presence of ozone stimulates marine organisms to migrate to land.
The atmosphere underwent continuous interaction with different terrestrial phenomena until it acquired a composition that currently consists of 99 percent hydrogen, oxygen, and argon. Today, the atmosphere not only serves to safeguard various physical phenomena occurring in space, but also functions as a remarkable regulator for thermodynamic, chemical, and biological processes that are crucial for the evolution and occurrence of Earth events, without which life as we know it would not be possible. This ongoing interplay between ocean temperatures, the protection provided by ozone from the sun’s harmful rays, and a relatively stable climate has enabled life to continue thriving.
I trust that this information will enhance your understanding of how the atmosphere formed and the processes involved.
The information provided in this article adheres to our editorial ethics guidelines. If you find any inaccuracies, please click here to report them.
Summary of the Article: Network Meteorology ” Meteorology ” Climatology ” Formation of the Atmosphere
The atmosphere, derived from the Greek words “atmos” meaning air and “sphaira” meaning ball, is the protective layer of air that surrounds and rotates with the Earth on its axis. It encompasses the outermost layer of the planet. The origins of the atmosphere remain a scientific enigma, and astronauts who have observed the Earth from space describe it as enveloped in a bluish haze.
The atmosphere is approximately 3,000 kilometers thick, extending from the Earth’s surface and gradually merging into outer space.
Structure of the atmosphere
The atmosphere is composed of air, which is comprised of various gases. The composition of atmospheric air includes nitrogen (78%), oxygen (21%), and other gases (1%). These other gases consist of carbon dioxide, helium, water vapor, ozone, krypton, xenon, argon, and other trace gases. Air possesses certain properties, such as transparency, colorlessness, odorlessness, invisibility, the ability to support combustion, as well as being highly compressible and elastic.
The temperature decreases until reaching the mesosphere, after which it starts to rapidly increase. At an altitude of 550 kilometers, the air temperature reaches an astonishing +1500 degrees.
The Significance of the Atmosphere
The significance of the atmosphere for Earth is immense since it consists of the air that provides the necessary conditions for the existence of all living organisms. Without this protective layer, life on our planet would be impossible. Some scientists even argue that humans do not inhabit the surface of the Earth, but rather reside at the bottom of an “ocean” of air.
The atmosphere is responsible for creating and shaping the planet we call home. However, human industrial activities, such as the release of carbon dioxide, soot, and dust, are gradually altering its composition. Currently, these changes are occurring without major consequences, but there will come a point where the atmosphere’s threshold will be exceeded.
Studying the Atmosphere
The study of the atmosphere is a complex matter and is being conducted worldwide. A specialized organization called the World Meteorological Organization (WMO), which includes Russia as a member, is devoted to the examination of the atmosphere. This examination is carried out using various tools, such as weather stations, balloons, rockets, and satellites. Balloons are utilized to determine the dimensions of the atmosphere. As a balloon ascends, it identifies the boundary between the troposphere and the stratosphere.
Weather refers to the conditions of the troposphere at a specific location and time.
What is the purpose of the Earth’s atmospheric layer?
Observations indicate that the density of air decreases rapidly as altitude increases. For example, at 5.5 km above the Earth’s surface, the air density is half of what it is at the Earth’s surface, and at 11 km it is four times less. As altitude increases, the air becomes increasingly thin.
In the outermost layers (hundreds and thousands of kilometers above the Earth), the atmosphere gradually transitions into the vacuum of space. The air envelope surrounding the Earth does not have a clearly defined boundary.
Due to the force of gravity, the gas density within a closed container varies throughout its volume. The bottom of the container experiences a higher gas density compared to the top, resulting in a difference in pressure. The pressure at the bottom is greater than at the top. However, this disparity in gas density and pressure is often negligible in many cases. Nevertheless, when considering an atmosphere that spans thousands of kilometers, this difference becomes significant.
Professional development course
Modern Approach to Education: Embracing Distance Learning

Continuing Education Course
Exploring Online Learning Platforms: Zoom, Skype, Microsoft Teams, and Bandicam
Continuing Education Course
Teaching Practices and the Teacher Professional Standard
Certificate and discount are offered to every participant who attends the training.
You can easily find relevant materials for any lesson by selecting your subject (category), class, textbook, and topic:
There are currently 5,608,243 materials available in our database.
The provided material is suitable for teaching purposes.
§43 Explaining the existence of Earth’s atmospheric layer
Participate in the most popular international distance learning contests
School Info Contests 2022
Every participant will receive a certificate and a discount on the training fees.
Additional Resources
These courses may also interest you:
Leave Your Feedback
- Date: 27.11.2018 | Downloads: 4505
- File Size: PPTX 2 MB
- Total Downloads: 210
- Rating: 5 out of 5
- Rate the Material:
Author of the Material
Moscow Institute of Professional
Retraining and Advanced Training
Teacher Training
Online Courses for Educators
We provide official certification upon completion!
Teachers Share Secrets for Successful Exam Preparation
The Ministry of Labor Proposes Simplified Process for Using Maternity Capital for Education
Kursk Authorities Implement Distance Learning for Students in Border Areas with Ukraine
Nearly Half of Students Experience Mental Aggression at School
10,000 Children from Luhansk and Donetsk Regions Seek Education in Russia
Expelled Students from Abroad Eligible for Free Study in Russia
In Belgorod Region, Classes in Schools and Kindergartens Cancelled Near Ukrainian Border
Gift certificates
The users who posted the materials on this site are responsible for resolving any disputes regarding the materials and their content. However, the site administration is willing to provide assistance in resolving any issues related to the site’s work and content. If you notice any unauthorized use of materials on this site, please notify the site administration through the feedback form.
All materials on the site are either created by the site’s authors or posted by users and are provided for reference purposes only. The legal authors hold the copyrights for these materials. Copying any materials from the site, in whole or in part, without the written permission of the site administration is prohibited! The views of the administration may not necessarily align with those of the authors.
The composition of the atmosphere is a mechanical mixture of various gases. Nitrogen makes up about 78% of its volume, while oxygen accounts for 21%. The remaining less than 1% is made up of gases such as helium, argon, krypton, and other inert gases. The levels of oxygen and nitrogen in the air remain relatively constant, as nitrogen does not readily combine with other substances and oxygen is replenished by plants, despite its active role in respiration, oxidation, and combustion.
Up to an altitude of approximately 100 kilometers, the proportion of these gases remains relatively unchanged. This is because the air is constantly being mixed.
In addition to these gases, the atmosphere also contains around 0.03% of carbon dioxide. This gas is typically found in higher concentrations near the Earth’s surface and is distributed unevenly. In areas such as cities, industrial centers, and regions with volcanic activity, the levels of carbon dioxide tend to increase.
In the atmosphere, there are always some impurities present, such as water vapor and dust. The amount of water vapor in the air depends on the temperature, with higher temperatures resulting in more vapor. The presence of water vapor in the air allows for various atmospheric phenomena, including rainbows and the refraction of solar rays.
Dust can enter the atmosphere through volcanic eruptions, sand and dust storms, and incomplete combustion of fuel at thermal power plants, among other sources.
The structure of the atmosphere changes with altitude, with the density being highest at the Earth’s surface and decreasing as you move upwards. For example, at an altitude of 5.5 km, the atmospheric density is half of what it is at the surface, and at an altitude of 11 km, it is four times less than at the surface layer.
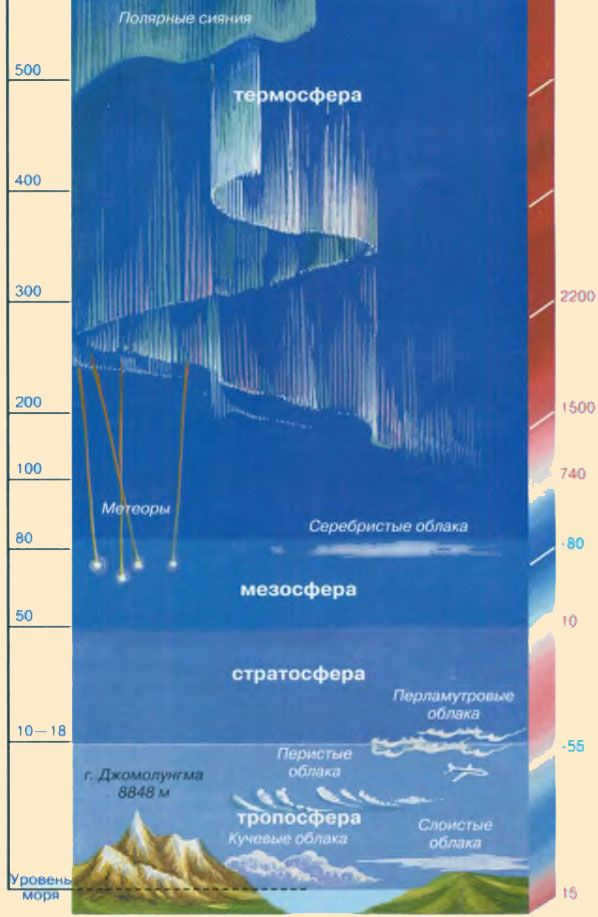
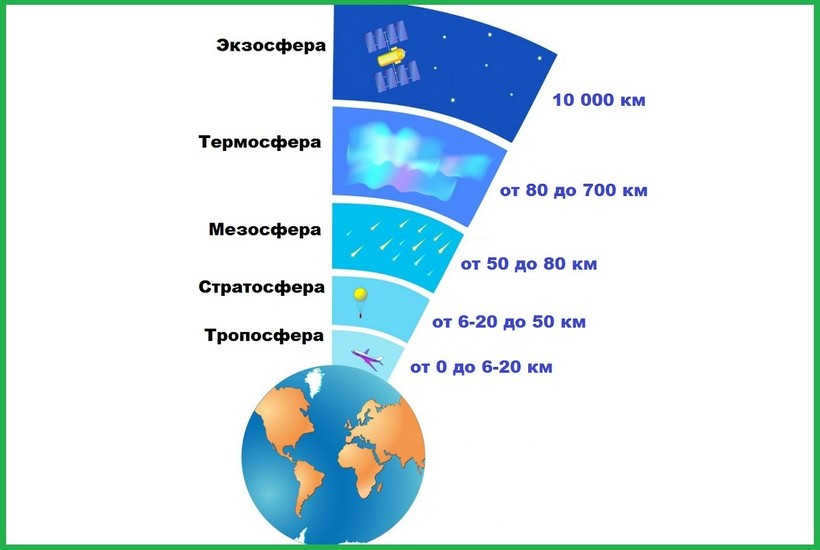
The atmosphere is divided into five concentric layers based on the density, composition, and properties of gases (see Figure 34).
Figure 34. Vertical structure of the atmosphere (atmospheric stratification)
The temperature of the air in the troposphere decreases by 0.6 °C for every 100 m increase in altitude and reaches -45-55 °C at its upper limit.
In the troposphere, the air is continuously blending and moving in various directions. It is only in this region that fog, rain, snowfall, thunderstorms, storms, and other meteorological phenomena can be observed.
2. The stratosphere is located above, reaching a height of 50-55 km. The stratosphere has minimal air density and pressure. The thin air consists of the same gases as the troposphere, but with a higher concentration of ozone. The highest concentration of ozone is found at altitudes of 15-30 km. As altitude increases, the temperature in the stratosphere also increases, reaching 0 °C and above at its upper boundary. This is because ozone absorbs the short-wave part of solar energy, resulting in heating of the air.
3. Above the stratosphere, we find the mesosphere, which extends up to 80 km. In the mesosphere, the temperature decreases once again, reaching -90 °C. The air density in this region is 200 times lower than at the Earth’s surface.
In the ionosphere, the occurrence of auroras leads to the generation of powerful electric currents, which in turn result in disturbances to the Earth’s magnetic field.
5. Beyond 800 km lies the outermost layer known as the exosphere. In this region, individual particles move at a speed close to the critical velocity of 11.2 mm/s, allowing them to overcome the gravitational pull of the Earth and venture into outer space.
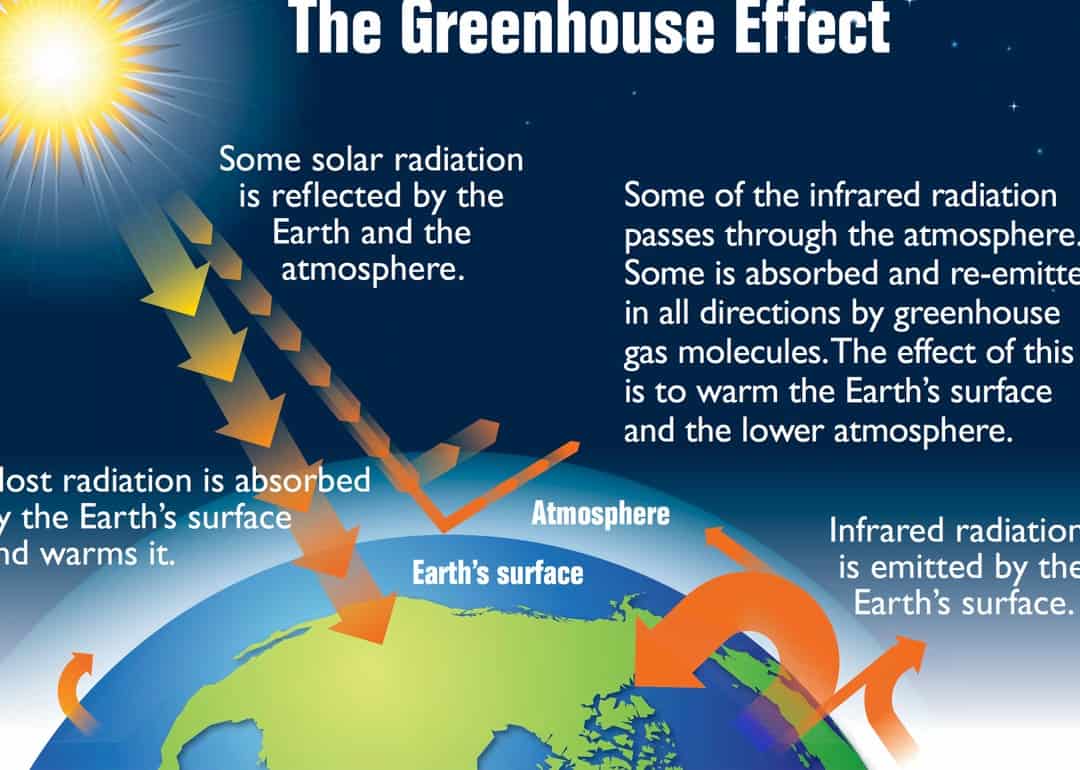
The atmosphere plays a crucial role in the existence of our planet. Its significance cannot be overstated, as without it, the Earth would be devoid of life. The atmosphere acts as a protective shield, shielding the Earth’s surface from extreme temperature fluctuations. It can be likened to the function of greenhouse glass, allowing sunlight to penetrate while preventing heat from escaping.
The atmosphere provides protection against the Sun’s short-wave and corpuscular radiation, safeguarding living organisms. It serves as the environment where various weather phenomena take place, which are closely tied to all human activities. The examination of this protective layer is conducted at meteorological stations. Meteorologists diligently observe the state of the lower atmosphere day and night, regardless of the weather conditions. They record temperature, pressure, air humidity, cloud cover, wind direction and speed, precipitation, as well as electrical and sound events in the atmosphere four times a day, and at many stations, even hourly. Meteorological stations can be found in various locations, ranging from Antarctica and humid tropical forests to high mountains and vast tundra regions. Additionally, observations are carried out on the oceans from specifically designed ships.
Observations in the Earth’s atmosphere have been conducted since the 1930s. Radiosondes were introduced during this time, which are instruments that are launched into the atmosphere and can reach heights of 25-35 km. These radiosondes transmit data on temperature, pressure, air humidity, and wind speed back to Earth using radio equipment. In addition to radiosondes, meteorological rockets and satellites are now commonly used for atmospheric research. Satellites are particularly useful as they have television installations that can capture and transmit images of the Earth’s surface and cloud formations.
2. Heating of the Atmosphere
The primary source of heat that warms the surface and atmosphere of the Earth is the sun. Although there are other sources such as the moon, stars, and the Earth’s internal heat, their contribution is so minimal that they can be disregarded.
The sun emits an immense amount of energy into space in the form of heat, light, ultraviolet, and other rays. This collective energy is known as solar radiation. The Earth only receives a minuscule fraction of this energy – one two billionth part – yet it is sufficient not only to sustain life but also to drive various processes in the lithosphere, as well as physical and chemical phenomena in the hydrosphere and atmosphere.
There are three types of solar radiation: direct, scattered, and total.
On a clear, cloudless day, the Earth’s surface receives its primary source of heat from direct radiation, which is felt as warm or hot sunlight.
As the sun’s rays pass through the atmosphere, they are reflected by air molecules, water droplets, and dust particles, causing them to deviate from their original path and scatter. The more cloudy the weather, the denser the cloud cover, and the greater the amount of scattered radiation in the atmosphere. In highly dusty conditions, such as during dust storms or in industrial areas, scattering reduces radiation by 40-45%.
The significance of scattered radiation for the Earth is immense. It provides illumination for objects in shadow and also contributes to the color of the sky.
The distribution of radiation on the Earth’s surface is significantly influenced by the Earth’s spherical shape and the tilt of its axis relative to the orbital plane. In equatorial and tropical latitudes, the sun remains high above the horizon throughout the year. In middle latitudes, the sun’s height varies depending on the time of year, and in the Arctic and Antarctic, it never rises very high above the horizon. As a result, the sun’s rays are scattered less in tropical latitudes, resulting in a higher concentration of radiation per unit area of the Earth’s surface compared to middle or high latitudes. Therefore, the latitude of a location affects the amount of radiation it receives, with less reaching the Earth’s surface as you move farther away from the equator.
1. What prevents the molecules of atmospheric gases from falling to the Earth?
The gas molecules in the Earth’s atmosphere are subject to the force of gravity, just like all other objects. However, due to their continuous and random motion, these gas molecules do not leave the Earth. As a result, an atmosphere, which is an air shell, is formed around the Earth as a result of the combined effects of gravity and the disorderly movement of gas molecules.
2. What prevents the gas molecules that compose the atmosphere from escaping the Earth?
A spaceship needs to reach a velocity of 11.2 km/s, known as the second cosmic speed, in order to leave the Earth and travel into space. This is the same speed that a gas molecule must attain to escape the Earth’s atmosphere and venture into outer space. However, the majority of molecules in the Earth’s air envelope have velocities much lower than this cosmic speed, resulting in only a small number of molecules actually managing to break free and enter outer space.
Does the density of the atmosphere change as altitude increases?
As altitude increases, the density of the air decreases. This is because the higher you go above the Earth’s surface, the smaller the column of air above, resulting in less weight and pressure. Consequently, the air becomes thinner the higher up you go.
At a height of 5.5 km above the Earth, the density of air is only half of what it is near the Earth’s surface. At an altitude of 11 km, the density is four times less.
The Earth’s atmosphere stretches out to several thousand kilometers in height.
The density and pressure of the air vary greatly at different altitudes.
In the upper layers of the Earth’s atmosphere, it gradually transitions into airless space.
The Earth’s atmosphere does not have a distinct boundary.
4. What is the relationship between the density of the atmosphere and temperature?
As air is heated, it expands and becomes lighter, meaning it becomes less dense and the atmospheric pressure decreases.
Conversely, when air cools, it contracts and becomes heavier, meaning it becomes more dense and the atmospheric pressure increases.

At present, there are 58,741 educational establishments that qualify for extra cumulative savings (ranging from 2% to 25%). To determine the specific discount applicable to all staff members at your educational institution, please access your personal Infoworks account.


Professional development course
Organizing project-based research activity while studying physics courses in the implementation of the Federal State Standard.
We have the option to include your educational institution’s discount along with this discount (depending on how many of your colleagues have taken Infowrok courses).
Currently, 58,741 educational institutions are eligible for additional cumulative discounts (ranging from 2% to 25%). To determine the discount applicable to all employees of your educational institution, please log in to your personal Infoworks account.
Continuing Education Program
Specifics of preparing for the Physics section of the Unified State Exam under the implementation of the Federal State Educational Standard for Secondary Education
In addition to this offer, we also provide a discount for your school (the amount depends on the number of your colleagues who have completed Infoworks courses).
Currently, 58,741 educational institutions are eligible for additional discounts ranging from 2% to 25%. To find out the exact discount available for all staff members of your educational institution, please log in to your personal Infoworks account.




The Theory of Relativity: Special and General
Summary of the presentation by individual slides:

Slide 1: The Existence of Earth’s Atmosphere
Presented by Darina Igorevna Lomivorotova, a physics teacher at MBOU “School № 77” Kemerovo

ατμος “atm” – gas
δφαίρα “sphere” – orb
ATMOSPHERE

Slide 3: The composition of the atmosphere is primarily made up of various gases. The most abundant gas is nitrogen, accounting for 78% of the atmosphere’s composition. Oxygen is the second most abundant gas, making up 21% of the atmosphere. Carbon dioxide, although present in much smaller quantities, still plays a crucial role in the atmosphere, accounting for 0.03% of its composition. Inert gases also contribute to the atmosphere, making up 0.93% of its composition.
The atmosphere serves as the Earth’s protective air envelope.

Slide 4: The atmosphere on Earth exists due to various layers. These layers include the troposphere, stratosphere, mesosphere, and thermosphere. Each layer has its own unique characteristics and plays a crucial role in maintaining the Earth’s climate and weather patterns.


In Slide 5, we can observe the view of the Earth’s atmosphere from the cockpit of a spacecraft as witnessed by Soviet cosmonaut Hermann Titov.
German Titov had the opportunity to see the Earth’s atmosphere firsthand from the cabin of a spaceship.

Slide number 6: The sensation of air pressure is not felt by humans until they reach the age of 50! This is because we inhabit the depths of the vast air ocean, where the weight of the atmosphere constantly surrounds us. Despite this, we do not perceive the immense pressure that each of us carries, equivalent to a staggering 10 tons on our shoulders!

On slide 7, we will explore the concept of air weight by conducting an intriguing experiment. To carry out this experiment, we will require the following materials: 1. Two balloons that are identical in size and shape. 2. A wire hanger. 3. Two plastic clothespins. 4. A horizontal handrail. 5. A needle.


Slide 8: The force of gravity affects air in the same way it affects any other object on Earth. As a result, air possesses weight.
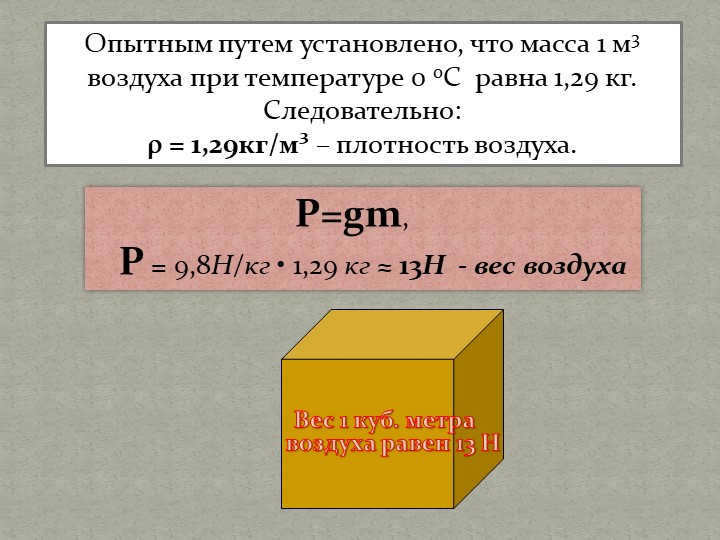
Slide 9: The weight of one cubic meter of air is determined to be 13 N. Through experimental observations, it has been found that the mass of one cubic meter of air at a temperature of 0°C is approximately 1.29 kg. Therefore, the density of air, denoted by ρ, is equal to 1.29 kg/m³. Using the formula P=gm, where P represents pressure and g represents the acceleration due to gravity (9.8 N/kg), we can calculate that the weight of the air is approximately 13 N.

Slide number 10
The molecules of the gases composing the atmosphere are in constant and disordered motion. Thus, they are unable to descend to the surface of the Earth.
In order to completely escape the Earth, a molecule must possess an exceedingly high velocity (at least 11.2 km/s). This velocity is known as the second cosmic speed. The majority of molecules in the Earth’s atmospheric envelope possess velocities substantially lower than this threshold.
The disordered motion of molecules and the influence of gravity upon them lead to the formation of an air shell, or atmosphere, which causes the gases to “float” in space surrounding the Earth.
The atmosphere lacks a distinct boundary.
What is the reason behind the existence of the Earth’s atmospheric envelope?

11th slide
Reviewing the topic that has been studied.
1. What is the reason behind atmospheric pressure?

Slide number 12 aims to further solidify the knowledge on the subject matter.
2. What is the reason behind the existence of the Earth’s atmospheric layer?

Slide number 13
Review and consolidation of the material covered.
Question 4: What is the mass of air occupying a volume of 1 cubic meter?


1. Are gas molecules in the atmosphere attracted to the Earth?
Yes, they are attracted.
2. Why don’t atmospheric gas molecules fall to the Earth’s surface?
The molecules are constantly moving.
3. Why don’t atmospheric gas molecules escape from the Earth?
Their speed is lower than the second space velocity.
4. What is the value of the second space velocity for the Earth?
11.2 km/s.
5. Do molecules in Earth’s atmosphere escape into outer space?
Yes, but only a small fraction of the molecules do.
6. Why do air molecules “float” above the Earth?
This is because of the combination of chaotic motion and gravity.
7. Choose the correct statement.
The density of air decreases rapidly as altitude increases.

Slide 17, Paragraph 43
The hypothesis suggests that the Moon once had an atmosphere but gradually lost it. How can we explain this phenomenon?
Assignment
Summary of the Document:
This is a physics presentation for 7th grade discussing the reasons behind the existence of Earth’s air shell.
- Evidence to be included in the portfolio
- Unlimited access for 99 rubles
- Over 3,800 video lectures available for everyone
Not everyone is aware of what the atmosphere is and its significance for Earth. It is worth noting that all planets in the solar system, except Mercury, have atmospheres. If the atmosphere did not exist, life would not be possible on our planet.
This article will explore the concept of the atmosphere.

Understanding the Atmosphere
The atmosphere of our planet Earth, derived from the Greek words “atmos” meaning vapor and “sphaera” meaning ball, refers to the gaseous envelope that surrounds our planet. It is one of the geospheres and covers the hydrosphere and part of the Earth’s crust on its inner surface, while blending into the near-Earth portion of outer space on its outer surface.
The atmosphere is the region surrounding our planet where the gaseous medium rotates along with the Earth as a cohesive unit. This means that there is no clear distinction between the atmosphere and outer space.
However, there is a hypothetical boundary with space known as the “Karman Line,” which is located approximately 100 kilometers above sea level. It should be noted that the composition of the Earth’s atmosphere, by volume, is as follows:
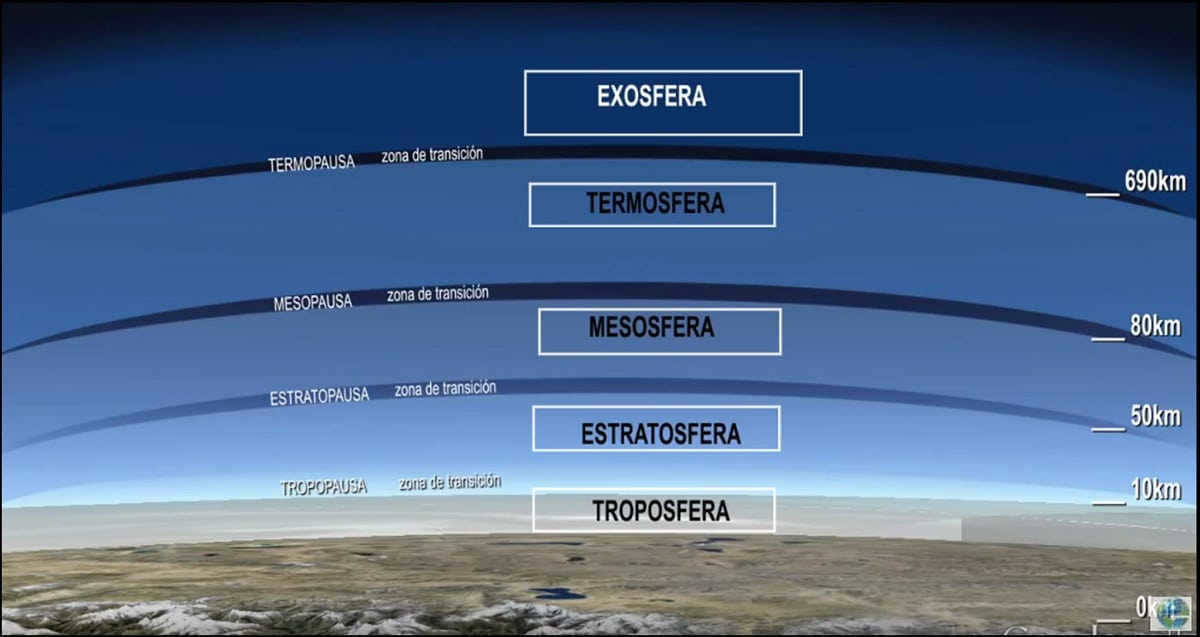
The composition of the atmosphere includes various gases, which comprise a minuscule proportion in terms of percentage. Furthermore, the Earth’s atmosphere is categorized into 5 distinct layers:
- The troposphere, situated at an elevation of 8-18 km, with its lowermost layer at an altitude of 1-2 km.
- The stratosphere, commencing at an altitude of 11-50 km.
- The mesosphere, located within the range of 50-90 km.
- The thermosphere, positioned at an altitude of 500-800 km.
- The exosphere, which constitutes an exceedingly rarefied outer layer of the atmosphere found at an elevation of 500-1000 km.
It should be noted that the progression from one layer to another is consistent, which is why geography identifies intermediary phases: tropopause (between the troposphere and the stratosphere), stratopause (between the stratosphere and the mesosphere), mesopause (between the mesosphere and the thermosphere), and thermopause (between the thermosphere and the exosphere).
Now you have a clear understanding of what the atmosphere is. If you found this article informative, feel free to share it on your social media platforms. And if you enjoy learning interesting facts about nature in general, be sure to subscribe to interesnyefakty.org.
Did you know?
Here are some related facts:
Having read the article on the atmosphere, I found it to be a fascinating source of information. It greatly expanded my understanding of the composition and proportions of gases that make life on Earth possible. It is truly remarkable that no other planet in our solar system possesses such a unique and essential atmosphere. The atmosphere acts as a shield, enveloping our planet and safeguarding it from extreme temperatures, radiation, space debris, and other potential threats. Not only do living organisms rely on the atmosphere, but they also play a crucial role in maintaining its balance through the cycling of oxygen and carbon dioxide. The gravitational force of the Earth holds the atmosphere together, while the Earth’s magnetic field protects it from the harmful effects of solar wind. As one Bible writer wisely stated, “Every house is arranged by someone; and he who arranged everything is God.” The existence of our vast and awe-inspiring “house” – the Earth – clearly points to the existence of a highly intelligent and skilled designer and builder, that is, God.
Ever since I was a young child, I have been captivated by the heavens above: the mesmerizing stars in far-off galaxies, the ever-changing clouds, and the radiant sun. In this article, I aim to divulge the most captivating pieces of information that I have learned through my geography classes and countless hours spent engrossed in various books and encyclopedias.

The vast atmosphere consists of tiny particles
Undoubtedly, everyone is aware that the term “atmosphere” refers to the planet’s air envelope. However, not everyone is acquainted with the fact that its length, estimated by scientists, is approximately 2500-3000 km, yet its boundaries remain indeterminate. The atmosphere comprises a plethora of diverse substances, with some being particularly prominent:

- The atmosphere on Earth contains mainly nitrogen, which makes up over 75% of it;
- Oxygen, on the other hand, is four times less abundant than nitrogen and makes up 21% of the atmosphere;
- In addition to nitrogen and oxygen, the atmosphere also contains other gases and water vapor.
Exploring the Atmosphere
Scientists from various fields are actively researching the nature and purpose of the atmosphere. They utilize state-of-the-art instruments at meteorological stations to gain insights into this enigmatic phenomenon. In addition, the launch of artificial satellites and rockets has greatly contributed to our understanding of the atmosphere, allowing us to unravel its secrets and expand our knowledge about this fascinating entity.

Curious facts about the atmosphere
Let me share a few intriguing facts about the atmosphere.
- According to modern scientists, our planet might have lost its atmosphere multiple times in the past.

- If Earth didn’t possess an atmosphere, it would be unsuitable for human life due to extreme cold.
- The atmosphere contributes to the formation of acid rain, which has a negative impact on organisms and the environment.
- Interestingly, certain layers of the atmosphere can support the existence of living organisms like bacteria.

Undoubtedly, numerous individuals possess a curiosity as to the origin of the white streaks that linger in the wake of an airplane’s movement. The explanation is quite straightforward: the combination of cold and hot gases occurs as a result of the interaction between the fuel and the atmosphere, ultimately producing these peculiar lines.
The atmosphere serves as the earth’s air envelope, or rather, the celestial envelope of a celestial body that encompasses and maintains gravity around it.
From a young age, I possessed a fascination with the field of geography. It appeared to me that the knowledge imparted to us in school was insufficient. Consequently, at the age of seven, I commenced taking supplementary geography lessons. It was during these sessions that I was enlightened about the atmosphere. This newfound knowledge is something I wish to impart upon you.

The atmosphere is a layer of air that surrounds the entire surface of our planet. It extends to a distance of 3000 km from the surface. The lower boundary of the atmosphere is the surface of the earth, while it has no upper boundary. The gravitational force of our planet holds the air at the surface, and the continuous movement of air particles prevents them from falling down.
What would occur if there was no atmosphere?
The existence of life on our planet is reliant on more than just the earth itself. What is the significance of the atmosphere in this regard? The atmosphere plays a crucial role as it acts as a source of oxygen. It is common knowledge that not only humans but also plants require oxygen to survive. Additionally, the atmosphere serves as a protective filter that absorbs ultraviolet light, shielding us from scorching harmful rays and excessively high temperatures. Moreover, the atmosphere acts as a barrier against radiation. To put it simply, without the presence of an atmosphere, our planet would experience extreme cold and extreme heat, rendering life impossible.
WHAT PREVENTED THE ATMOSPHERE FROM BEING DRAWN INTO THE COSMIC VACUUM?
A vacuum refers to a region devoid of matter, characterized by extremely low density and pressure. The cosmic vacuum, found in outer space, embodies these properties, as there are only a few hydrogen atoms per 1 cm³ in this vast expanse. Consequently, the cosmic vacuum closely resembles a modern physical vacuum.
Conventional scientific understanding dictates that our atmosphere is sustained by the universal law of gravitation, which prevents it from escaping into space. However, it is worth pondering whether we should unquestioningly trust scientists. It appears that the vacuum is actually much more powerful than the gravitational field.
This raises the perplexing question: why isn’t our atmosphere being pulled into the cosmic vacuum? Unfortunately, this query is likely to remain unanswered. Perhaps our perception of the world order is not as accurate as we have been led to believe.
2) The utilization of eco-friendly fuels
3) Enhancing overall awareness regarding the environment

This article aims to demonstrate the significance of the atmosphere for sustaining life on Earth.
During my childhood, I developed a keen interest in books from the “I’m learning about the world” series. It was in one of these books that I first encountered the concept of the atmosphere. Intrigued by this “phenomenon,” I embarked on a deeper exploration of its nature, structure, and purpose. In the following paragraphs, I will provide a comprehensive overview of my findings.

What is known as the atmosphere
The atmosphere is the envelope of air or gases surrounding a planet. The term is derived from two Greek words: “atmos”, meaning breath, and “sphere” – in its literal sense. One might assume that the atmosphere would escape into space due to its extremely low weight, but this is not the case, as the planet’s gravity keeps the atmosphere in place.
The atmosphere of our planet
The atmosphere of our planet is breathable. It is composed of:

- Nitrogen makes up approximately 77% of the atmosphere, making it the most abundant element;
- Oxygen accounts for 21% of the atmosphere;
- Argon makes up about 1% of the atmosphere;
- The remaining 1% of the atmosphere is comprised of other elements.
There are various substances found in the atmosphere, including carbon dioxide, methane, neon, xenon, and many others.
Atmospheres of different planets
Planet Earth stands out as a unique celestial body, being the only known planet that fully supports life. Other planets cannot make this claim. Take Venus, for instance, which possesses a dense atmosphere primarily composed of carbon dioxide, resulting in extreme heat and high atmospheric pressure. Mercury, the closest planet to our sun, has an almost non-existent atmosphere. On the other hand, there are gas giant planets that consist of an outer layer of gas. For instance, Saturn is mostly composed of hydrogen and helium.

Ways to Safeguard the Atmosphere
To ensure the atmosphere on Earth remains conducive to life, it is crucial to prioritize its preservation. Here are some measures you can take:

- Implementing specialized treatment facilities within industrial plants to purify the contaminated atmosphere;
- Adopting recycling methods for waste management instead of resorting to incineration;
- Promoting the use of bicycles and walking for transportation purposes.
If we are able to eliminate flammable fuels and cease the destruction of the atmosphere through emissions, our society will endure for an extensive duration.
During my academic years, I possessed a profound fascination with geography, particularly focusing on the subject of the atmosphere. Today, I will endeavor to provide assistance in comprehending this straightforward yet captivating concept. The majority of individuals possess a basic understanding of what the atmosphere entails on a logical level. My aim is to explain it in a simple and accessible manner, enabling you to apply the acquired knowledge effortlessly.

Explanation of the atmosphere
To put it in simpler terms, the atmosphere can be described as the protective layer of gases that surrounds a planet and moves along with it. This layer is found in almost all major planets in our solar system. Here are the different compositions of the atmospheres in our solar system:

- The composition of the Earth’s atmosphere is primarily made up of oxygen (1/5) and nitrogen (4/5);
- Mercury, on the other hand, has an extremely thin gas layer that is virtually non-existent;
- When it comes to the giant planets Uranus, Saturn, Jupiter, and Neptune, they all have a similar composition in their gas envelopes, containing helium, hydrogen, and methane;
- Venus, on the other hand, has an atmosphere that is almost entirely composed of carbon dioxide;
- As for Mars, it has the most similar atmosphere to Earth’s, although it lacks enough oxygen to support life, and is instead dominated by carbon dioxide.
Composition of the Earth’s Atmosphere
Let’s explore more about our home planet. In a nutshell, the Earth’s atmosphere is comprised of 5 distinct layers, each with its own unique characteristics. These layers are separated by transitional regions, adding to the complexity of our atmosphere.
I will highlight some of the most fascinating aspects. Take, for instance, the lowest layer known as the troposphere, which contains an abundant supply of oxygen that is essential for our breathing. Moving up, we encounter the stratosphere, where a significant amount of ozone gas acts as a protective shield against harmful ultraviolet rays. The ionosphere, on the other hand, is filled with ionized atoms that reflect radio signals. Finally, the exosphere represents a gateway to the vastness of outer space.
Besides its role in containing oxygen, the atmosphere effectively manages to prevent detrimental elements such as space dust and asteroids from reaching the Earth.
Currently, it has become trendy (a phrase I personally enjoy using) to refer to “our own atmosphere.” However, it is important to consider the geographical definition of the atmosphere. Can it be sensed or physically interacted with? Does an atmosphere exist on other planets?

Understanding the Concept of Atmosphere
When attempting to convey the idea of atmosphere to a child, I would describe it as a layer of vaporous substance that envelops our planet, Earth. This enveloping layer, also known as the air shell, consists of a unique blend of various gases:
Responsibilities of the Earth’s Atmosphere
The Earth’s atmosphere can be compared to a multilayered pie, consisting of five distinct layers. Each layer varies in thickness depending on the geographic latitude and is responsible for specific functions:

- The troposphere accounts for 80% of the air mass and is where precipitation is formed and air movement occurs;
- The stratosphere acts as a shield, trapping ultraviolet radiation from the sun with a layer of ozone;
- The mesosphere has an air density that is 200 times less than that of the Earth’s surface;
- The ionosphere is responsible for the appearance of polar lights and the occurrence of sharp fluctuations in the magnetic field;
- The exosphere is where gases move at such high speeds that they can escape into outer space.
Atmospheres on other planets
Every planet has its own unique atmosphere. For instance, Venus possesses a gas envelope comprised of carbon dioxide and nitrogen, which continuously circulates and rotates once every four Earth days. Mars, on the other hand, has a vastly different atmosphere compared to Earth, characterized by lower pressure, a weak magnetic field, and a different chemical composition. The troposphere on Mars extends from 20 to 30 km in height, whereas on Earth it ranges from 8 to 18 km (depending on the geographic latitude). Uranus, Jupiter, and Saturn all share a similar composition in their atmospheres, consisting mainly of hydrogen and helium. Interestingly, Uranus holds the title for having the coldest atmosphere in the entire solar system.

The gaseous envelope of the planets can undergo changes due to various factors, but initially, the primary causes are the chemical and temperature properties of the Sun during the planets’ formation. As a result, throughout the history of its formation, the Earth has existed in three distinct states.
Even in my childhood, my grandmother (an experienced geographer) explained this topic to me in a simple and clear manner. The atmosphere surrounds our planet from all directions, and it consists of a gaseous substance where various phenomena occur, such as electrical and optical phenomena. There are many more intriguing aspects to be discussed about this “air ocean,” as the atmosphere is figuratively called.

The Definition of the Atmosphere
The atmosphere serves as the primary source of photosynthesis and respiration for plants and animals. Additionally, it acts as a crucial barrier against infrared radiation, effectively safeguarding the Earth’s thermal equilibrium. Moreover, the atmosphere plays a pivotal role in protecting our planet from potential meteorite impacts. The movement of air, as well as the changes in weather patterns and climate, all occur within the atmosphere. Valuable data about the ongoing physical processes within the atmosphere is provided by meteorological posts, observatories, and stations.


The distribution of meteorological stations across a specific area is essential. It is important to have standardized methods of work and uniform instruments. Interruptions in observations should be avoided. Adhering to these guidelines will foster scientific research on the atmosphere and enable the population to stay informed about climatic changes.
Similar to many schoolchildren, I too aspired to become an astronaut. My decision was influenced by a significant event in human history – the first manned spaceflight, accomplished by our fellow countryman Yuri Gagarin. From that point onward, I developed an interest in the concept of the atmosphere, its composition, and the potential consequences of its absence.


The Earth’s Atmosphere
The Earth is composed of various layers, some of which are familiar from geography lessons:
And then there’s another layer, the atmosphere, which is the Earth’s layer of air. It completely surrounds the planet. This layer is approximately 3,000 kilometers thick. The lower part of this layer is the planet’s surface, and it is believed that this layer has no upper boundary. Its continuation is considered to be outer space. As we learn in physics lessons, the air is held in place by the Earth’s gravitational force, so you might wonder why it doesn’t all fall to the ground. This is because the particles are constantly in motion, preventing them from falling to the ground.

Earth’s atmosphere: Different Layers
The first layer, known as the troposphere, extends up to 18 kilometers above the Earth’s surface. However, this height varies depending on the location. At the equator, it reaches its maximum thickness, while at the poles, it is around 8-10 kilometers. The troposphere holds approximately 80% of the entire air volume. It is the layer we find ourselves in when we travel by plane, experience rainfall, and encounter strong winds.
Continuing above the troposphere, we encounter the stratosphere. In this layer, the concentration of air is significantly lower compared to the previous layer.

Next, we have the higher regions of our world:
What if we didn’t have the atmosphere
Our globe is presently the sole known location that is appropriate for human existence. Humans have adapted fairly well due to the weather conditions and environment of our planet.
It can be deduced from the information provided that if there were no atmosphere, the Earth would be subjected to extreme temperatures, including freezing cold and scorching heat. Additionally, the planet would be vulnerable to harmful radiation and ultraviolet rays. In such conditions, life as we know it would be utterly impossible.

Upon the cold water being shut off in our residence, I once again became aware of the immense significance of water to our daily lives. From a young age, we were instilled with the importance of conserving water, yet not everyone heeded this advice. Upon delving into specialized literature, I came to the realization that certain countries are currently grappling with issues stemming from the scarcity of potable water. Just under half of the global population is directly affected by this pressing issue.

The Issue of Water
Currently, only three percent of the Earth’s water is suitable for drinking. According to scientific projections, by the middle of the twenty-first century, approximately four billion individuals will experience a scarcity of drinking water.

The Pope has proposed that the scarcity of clean water could potentially be a driving force behind conflicts and wars. The lack of access to fresh water is becoming a growing concern due to various factors such as population growth, increasing industrial water demands, the impacts of climate change, and the pollution caused by chemical and other manufacturing facilities.
- Rapid population growth: The increasing global population puts a strain on the available water resources, leading to scarcity.
- Industrial water demand: The rising demand for water in industrial processes further exacerbates the scarcity issue.
- Climate change: The changing climate patterns can disrupt the water cycle, affecting the availability of fresh water sources.
- Atmospheric pollution: The pollution generated by chemical and other plants can contaminate water sources, rendering them unsafe for consumption.
These are just a few examples of the reasons why the scarcity of fresh water is being felt worldwide. As the scarcity intensifies, the price of water is likely to increase, leading to potential political instability and conflicts.

Ways to Address the Water Crisis
Currently, there are several solutions available to tackle the water crisis. One approach is the construction of reservoirs. Across the globe, there are approximately thirty thousand reservoirs. However, it is not feasible to construct these massive structures due to concerns such as land deflection, changes in groundwater levels, and potential flooding. Another promising avenue is the desalination of seawater, which can provide a significant amount of freshwater. Additionally, the utilization of icebergs can be a viable solution. By transporting them and extracting drinking water from them, we can alleviate the water shortage. Another option is to discharge accumulated water into rivers.
The scarcity of clean water in Russia is not a pressing issue. The country boasts an abundance of water resources, with nearly three million rivers and an equivalent number of lakes. One prominent example is Lake Baikal, a massive reservoir renowned for its pristine water.

Based on the information presented in this article, it can be concluded that it is imperative for humanity to exert maximum efforts in order to safeguard the resources from which potable water can be obtained. Additionally, there is a need to explore more cost-effective approaches to address the scarcity of fresh water.
Once, I stumbled upon a fascinating article that discussed the essence of the existence of the various planets within our solar system, delving into the intricate details of Jupiter, Saturn, and our very own Earth. What caught my attention was its focus on the intricate chemical makeup of these celestial bodies. This piqued my curiosity, prompting me to delve deeper into an extensive exploration of the atmospheres surrounding these cosmic entities. An atmosphere serves as a protective shield encompassing a planet, primarily consisting of various gases. It is this shield that shields us from hazardous solar radiation and enables our ability to breathe and flourish.

Atmosphere of Earth
The attractive force of any celestial object pulls gaseous substances towards it, and the Earth is not exempt from this phenomenon. It is worth mentioning that we are fortunate to possess an atmosphere that consists primarily of nitrogen and oxygen, as these gases are crucial for supporting carbon-based life forms. While nitrogen and oxygen are the dominant chemical elements, there are also other substances present:

- Hydrogen gas.
- Krypton element.
- Xenon gas.
- Neon gas.
- Carbon dioxide gas, and so on.
One notable feature of the Earth’s atmosphere is the existence of various layers, such as the troposphere, the stratosphere, and the mesosphere. These different zones play a crucial role in shaping our planet’s environment and supporting life. The atmosphere directly influences natural processes and has a significant impact on animal life. It also plays a vital role in determining weather patterns and climate conditions on different continents. Moreover, our bodies have adapted to function optimally under specific atmospheric pressures. When individuals venture into the vacuum of outer space, adapting to the new conditions becomes extremely challenging.

The largest atmosphere in the solar system
Jupiter has the largest and heaviest atmosphere in our solar system as it is primarily composed of gas. What makes it even more fascinating is that it is incredibly challenging to determine the exact boundary between the planet’s surface and its atmosphere, unlike Earth where this distinction is visibly clear. Personally, I find Jupiter to be one of the most enigmatic and intriguing planets to study in our solar system because it is difficult for us to comprehend how such a massive object can exist without a solid surface to stand on.
Why “Atmosphere”
The term “Atmosphere” originates from two ancient Greek words that mean “vapor” and “sphere.” Consequently, the layer of gas surrounding celestial bodies is referred to as the atmosphere. The boundary that separates this gas from the interplanetary space is somewhat indistinct. By the way, this space, which separates the planets, is not “empty,” but contains various components, including gas. Hence, it is customary to refer to the atmosphere as the region encircling a planet that moves in tandem with the planet.

Earth’s atmosphere
Within the atmosphere of our planet Earth, it is conventionally recognized that there are five primary layers:
Troposphere – the lowermost layerThe troposphere constitutes the lowest layer, with a thickness ranging from 8 to 18 kilometers. Above the troposphere lies the stratosphere layer, which has a thickness ranging from 8 to 50 kilometers, followed by the mesosphere with a thickness ranging from 50 to 80 kilometers. Finally, above these layers, there is a thermosphere, which can span up to 800 kilometers in thickness, and an exosphere dispersal layer, which can span from 800-1000 kilometers in thickness.

Within the stratosphere, the ozone layer is situated, and the preservation of this layer is currently a top priority for humanity. The presence of ozone is vital for maintaining the suitable conditions for life on our planet.
Creating an Artificial Atmosphere on Mars
Researchers are pondering the feasibility of generating an artificial atmosphere on our closest celestial neighbor, the planet Mars. Presently, Mars is an arid wasteland devoid of life. In theory, by raising the temperature at the Martian poles, the substantial reserves of ice and carbon dioxide on the planet would evaporate, resulting in a denser atmosphere and the possibility of oxygenation.

However, I completely concur that the primary issue lies in the absence of a robust magnetic field on Mars, akin to Earth’s magnetic field. There exist various propositions to address the magnetic field quandary on Mars, although they are all not the current undertakings.
At times, I yearn to marvel at the clouds. One gazes upon them, and they possess such diversity and beauty. It is awe-inspiring how simplistic air can fashion such exquisite forms. I became cognizant of its significance during my childhood. Nevertheless, it proves arduous to fathom that air is not merely an intangible entity but a comprehensive system. It is known as the atmosphere, and I shall elucidate its essence, step by step.

What is the Earth’s atmosphere
I’m certain that many individuals can recall the definition learned in school that the atmosphere represents the air envelope surrounding the Earth. It functions as a protective shield for our planet, filling the entirety of space. In terms of its boundaries, the air extends across the entire Earth in varying proportions. It is less prevalent in bodies of water and soil, while at higher altitudes, the percentage is noticeably higher. The gravitational field maintains the cohesion of the Earth’s atmosphere, and as it weakens, the concentration of air diminishes. It is evidently impossible to ascertain the upper limit of our atmosphere.

Composition of the Earth’s Atmosphere
The Earth’s atmosphere is not uniform. It consists of various layers, and I often liken it to a multi-layered cake to help with visualization.

- The first layer, known as the planetary boundary layer, is situated closest to the Earth’s surface and has a thickness of approximately 2 km. This layer is unique in that its state is intricately connected to various Earth processes.
- The troposphere, which is the lowermost layer of the atmosphere, contains the majority of the Earth’s air. Its thickness varies depending on the geographical location, with the widest area found at the equator. This is where the enchanting formations of water vapor known as clouds can be observed, which I find quite fascinating. I am grateful to the troposphere for these awe-inspiring sights!
- The region between the troposphere and the stratosphere is known as the tropopause. This layer is distinguished as a distinct section because the temperature of the air remains constant here.
- Located above the tropopause, the stratosphere is another important layer. It contains the ozone layer, which shields our planet from the harmful effects of cosmic rays.
- The stratopause is a well-known transitional state within the atmosphere.
- Following the stratopause is the mesosphere, which acts as a protective shield against meteorites that usually disintegrate upon entry.
- The thermosphere is where the mesmerizing natural phenomenon known as the aurora borealis takes place.
- The thermopause is the layer responsible for certain functions, as I’m sure you can imagine.
- The exosphere is situated approximately 500 km above the Earth’s surface. It is at this point that our atmosphere bids us farewell, as gravity can no longer retain its gases and particles, allowing them to embark on a voyage through space.