The atmosphere is composed of a combination of 9 essential gases that encompass the Earth’s surface. This mixture of gases forms the air, providing the necessary conditions for the sustenance of living organisms.
Which gases make up the Earth’s atmosphere?
As mentioned previously, the composition of the Earth’s atmosphere is not uniform – it consists of a mixture of various gases that are invisible and intangible. Here are a few examples:
Nitrogen and oxygen are the primary gases in the air mixture, comprising the majority of it. It should be noted that nitrogen is the most abundant gas on our planet and is also widely present on Uranus, Neptune, Pluto, and even in interstellar space.
Oxygen is the second most important gas after nitrogen. An interesting fact about oxygen is that it is toxic in its pure form, but a deficiency of it is also detrimental to health. The amount of oxygen in the air is precisely what is necessary for the comfortable existence of all organisms on our planet.
Advantages of air for living beings

Air is vital for the existence of all organisms on Earth, including humans. A person breathes in and out 20 thousand times a day. When you inhale, your body absorbs oxygen, which then quickly circulates through the bloodstream, providing every cell with the necessary energy to function. Subsequently, the processed oxygen is exhaled as carbon dioxide into the atmosphere. This process enables the exchange of heat between the human body and the surroundings.
Air plays a crucial role in the survival and well-being of animals, as it directly impacts their growth, development, reproductive abilities, size, age, and daily activities. Insufficient oxygen levels in the air can have severe consequences for animals, causing an increase in their breathing rate and blood flow, while simultaneously slowing down vital bodily processes. Consequently, animals may experience anxiety and develop serious illnesses when deprived of proper oxygen supply.
Plants also heavily rely on air for their existence. While they require oxygen for respiration, their nutritional needs are fulfilled by carbon dioxide. In the absence of oxygen, plants would be unable to germinate and grow from the soil. Oxygen is essential for all parts of a plant’s organism, including the roots, stems, and leaves. Moreover, the quality of a plant’s life is directly influenced by the amount of carbon dioxide present in the air, as higher concentrations result in improved plant growth and vitality.
The circulation of air in the atmosphere is of great significance for plants. When air moves in an upward direction, it facilitates the dispersal of seeds and pollen, while also creating an optimal temperature. Conversely, when the air moves horizontally, it can cause plants to become dehydrated and wilt.
Therefore, it can be confidently stated that air is an essential component for humans, animals, and plants. The quantity and quality of air are particularly crucial.
What are the physical characteristics of air?

So, the composition of atmospheric air consists of a mixture of over 9 gases, with the primary components being Nitrogen and Oxygen. Despite being invisible, intangible, and odorless, atmospheric air possesses several physical properties.
For instance, atmospheric air has a low thermal conductivity and allows sunlight to pass through easily. This is due to the air’s transparency, causing the heat from sunlight to affect visible objects in its surroundings rather than the air itself.
Furthermore, air occupies the space it fills. This can be easily demonstrated through a simple experiment at home. By filling a small container with water and placing an upside-down glass into it, a slight resistance can be felt. This resistance occurs because the water is unable to occupy the space already taken up by the air.
Air possesses weight, or in scientific terms, mass. This characteristic implies that it applies pressure on the objects surrounding it. According to scientific research, the Earth’s atmosphere exerts a pressure on an individual equivalent to 15 tons (approximately the weight of three trucks). Nonetheless, the human body also contains air, which creates internal pressure with the same magnitude. As a result, external and internal pressures are balanced, and individuals do not perceive the immense weight of the atmosphere.
Furthermore, air exhibits elastic properties, similar to that of a spring, allowing it to compress and subsequently regain its original shape. This phenomenon is also observed during the heating or cooling of air: as the temperature increases, air expands and rises, while it contracts and descends when the temperature decreases.
Air is a companion to humanity

In the course of daily existence, individuals frequently employ innovations that operate by virtue of the physical characteristics of the Earth’s atmospheric air. For instance, in antiquity, it was utilized in navigation: the wind propelled the sails, enabling ships to travel in the desired direction. Since ancient times, individuals have commenced constructing windmills, whose functionality is contingent upon the rotation of their blades propelled by the currents of atmospheric air.
The physical properties of air have become a valuable asset in modern industry. A prime example of this is the invention of the diving bell, which enables various underwater operations. The bell is submerged in water, but due to air resistance, it always contains oxygen, as demonstrated in the earlier glass experiment. This characteristic allows individuals to descend to great depths without the need for specialized equipment, ensuring their safety. This technology is frequently utilized for bridge repairs, sea and river vessel inspections and repairs, and aiding divers in deep-sea exploration.
In present times, humanity has discovered numerous practical uses for air in their daily lives. It is employed to inflate tires, wash cars, and clean rooms and clothing, effectively removing dust and small particles of dirt.
To sum up, the atmospheric air plays a vital role in sustaining life on Earth. It is composed of over 9 different gases and possesses various physical properties. Furthermore, it is extensively utilized by humans in their daily lives.
The atmosphere, which encompasses the planet, is crucial for the development of life on our planet. The distinctive composition of atmospheric air enables organisms to oxidize organic substances using oxygen and acquire energy for survival. Without this essential component, the existence of humans, animals, plants, fungi, and bacteria would be unattainable.
Importance for human beings
The atmosphere serves a crucial role for human beings, providing more than just oxygen. It enables humans to have the ability to see, interpret spatial signals, and utilize their senses. Our hearing, vision, and sense of smell are all influenced by the condition of the atmosphere.
Another significant aspect is the protection it offers against solar radiation. The atmosphere acts as a shield, trapping a portion of the sun’s rays within its shell. Consequently, only around 30% of the solar radiation ultimately reaches the Earth’s surface.
Currently, various chemicals such as oxygen, helium, argon, and nitrogen are being extracted from the atmosphere. Although this technology is still in the testing phase, it holds great potential for the future of the chemical industry.
The aforementioned facts may seem obvious, but the air environment plays a crucial role in both industrial processes and human economic activities:
- It serves as a vital chemical agent for combustion and oxidation reactions.
- It facilitates the transfer of heat.
Therefore, the atmospheric air is a unique environment that enables the existence of living organisms and the development of various industries. There is a close interaction between the human body and the air environment, and any disturbance to this interaction can have serious consequences.
Atmospheric pollution poses a significant environmental challenge in the 21st century. The release of toxic chemicals, organic matter, and pathogenic microorganisms alters the composition of the atmosphere. Similar to other components of the Earth’s geographical envelope, the atmosphere has the ability to naturally cleanse and regulate itself. However, it is uncertain when the capacity for self-purification will be fully depleted.
Gas composition
Which gases make up the atmosphere? The chemical makeup of atmospheric air remains relatively constant, serving as the most crucial indicator reflecting the state of the environment.
The atmospheric air comprises the following gases:
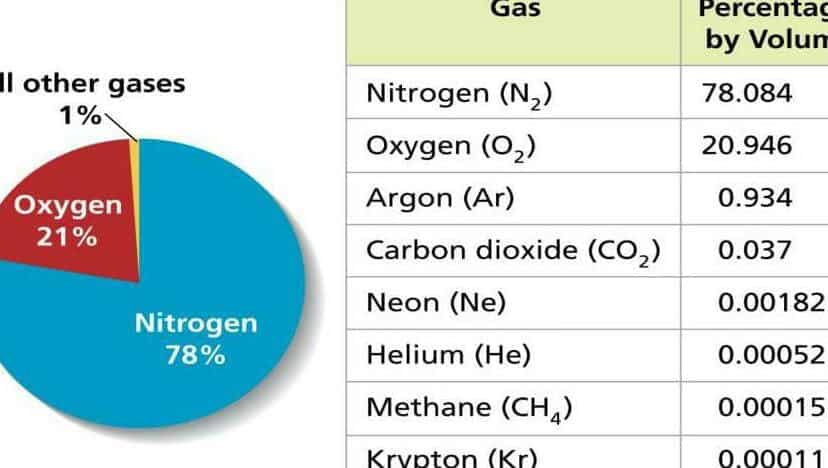
- Nitrogen – 78%.
- Oxygen – 21%.
- Water vapor – approximately 1.5%, with the exact value depending heavily on the climate zone and air temperature.
- Slightly less than 1% argon.
- 0.04% carbon dioxide
- Ozone.
And other gases that are a fundamental and unchanging component of the atmosphere. The gas makeup of the atmosphere remains intact thanks to the natural substance cycle. Oxygen is of utmost importance for human life as it is generated through the assistance of plants. Hence, researchers have successfully computed that a mere 3% reduction in oxygen levels can result in a complete cessation of all biological activities on Earth. Ozone is necessary to disperse oxygen and is also found in high concentrations in the upper stratosphere, forming the ozone layer that shields the Earth from solar radiation.
Consistency of Composition
The atmosphere has the ability to regulate itself and maintain a consistent composition. If its chemical makeup were to change, only bacteria would remain on Earth. Fortunately for humans, the atmosphere is capable of removing localized pollution.
This self-regulation is achieved through:
- Precipitation, which falls as rainwater and introduces pollutants into the soil.
- Chemical reactions that occur directly in the air involving oxygen and ozone. These reactions are oxidative in nature.
- Plants that oxygenate the air and absorb carbon dioxide.
However, no amount of self-regulation can completely eliminate the harm caused by industry. Therefore, the protection of atmospheric air has become increasingly important in recent times.

Air Quality Assessment
Air pollution occurs when substances that should not be present in the atmosphere enter it. Pollution can be natural or artificial, with natural pollutants being neutralized through the planet’s natural cycle of matter. On the other hand, artificial pollution is more complex and requires human intervention to address.
Natural sources of pollution include:
Artificial pollution, on the other hand, is caused by human activities. It can be classified into global pollution, which refers to emissions that can impact the overall composition or structure of the atmosphere, and local pollution, which pertains to changes in air quality within specific areas used for residential, occupational, or social purposes.
Atmospheric air hygiene is a crucial aspect of hygiene that focuses on assessing and managing indoor air parameters. This field emerged in response to the need for sanitary protection. The significance of atmospheric air in terms of hygiene cannot be overstated – when we breathe, all the impurities and particles present in the air enter our bodies.
The hygienic assessment of atmospheric air includes the following indicators:
- Physical properties of atmospheric air. This encompasses temperature (a common violation of SanPin in workplaces is excessively hot air), pressure, wind speed (in open areas), radioactivity, humidity, and other factors.
- Presence of impurities and deviation from the standard chemical composition. This provides an understanding of the suitability of atmospheric air for breathing.
- There are solid impurities present, such as dust and other microparticles.
- There is bacterial contamination present, including pathogenic and conditionally pathogenic microorganisms.
In order to assess the hygiene level, the measurements obtained at the four points are compared to the established standards.

Preservation of the Environment
Lately, environmentalists have been concerned about the state of the Earth’s atmosphere. With the advancement of industry, environmental risks are increasing. Factories and industrial areas not only harm the ozone layer by heating up the atmosphere and polluting it with carbon impurities, but they also compromise the air’s hygiene. That is why developed countries have implemented comprehensive measures to protect the air environment.
The main strategies for protection include:
- Enforcement of legislation.
- Development of guidelines for the placement of industrial areas, taking into consideration climate and geographical factors.
- Control of sanitary and hygienic conditions in enterprises.
- Regular monitoring of the composition.
Measures to protect the environment also include the establishment of green areas, the creation of artificial water reservoirs, and the implementation of buffer zones between industrial and residential areas. Conservation measures have been developed by organizations such as the World Health Organization (WHO) and the United Nations Educational, Scientific and Cultural Organization (UNESCO). State and regional recommendations have been based on these international guidelines.
Currently, there is an increasing focus on air hygiene. Unfortunately, the current measures are not sufficient to fully mitigate the anthropogenic impact. However, it is hopeful that with the development of more environmentally friendly production facilities in the future, the burden on the atmosphere can be reduced.
Air is a combination of various gases, primarily nitrogen and oxygen, which make up around 98-99% of the total composition. It also contains argon, carbon dioxide, and hydrogen, all of which contribute to the formation of the Earth’s atmosphere.
The Role and Importance of Air:
Air refers to the mixture of gases mentioned above. It is an essential element for sustaining life on Earth and plays a crucial role in maintaining the balance of our ecosystem.
In Russia, the Federal Law of 04.05.1999 No. 96-FZ, known as “On Atmospheric Air Protection,” provides a legal framework for preserving the quality of atmospheric air.
Atmospheric air is a vital component of the environment. It is a natural blend of gases found outside residential, industrial, and other structures. This type of air is an integral part of the habitat for humans, plants, and animals, supporting their survival and well-being.
The atmosphere envelops planet Earth, constituting the planet’s air cover. It is kept in place by the gravitational force of the Earth. Earth’s atmosphere safeguards life on Earth by pressurizing the planet, permitting the presence of liquid water on the planet’s surface, absorbing detrimental ultraviolet radiation emitted by the sun, heating the surface by trapping heat (known as the greenhouse effect), and minimizing temperature disparities between day and night (diurnal temperature change).
Air is indispensable for the normal survival of living organisms on Earth. Devoid of air, human existence becomes unviable. For humans, a crucial component of air is oxygen. Oxygen present in the air, during the process of respiration, enters the body’s cells and is employed in the process of oxidation, resulting in the release of vital energy required for life (metabolism, aerobic organisms).
If a person is deprived of food, they can survive for several weeks, while without water, they can only last for a few days. However, without air, a person can only survive for a few minutes, with the average person lasting around 1 minute and trained divers lasting around 5 minutes.
The total mass of air on Earth is approximately 5.13․10 15 tons, which exerts an average pressure of 1.0333 kg per 1 cm 3 at sea level on the Earth’s surface.
Characteristics of air:
The properties of air are that it is colorless, tasteless, and odorless, and it is completely transparent.
Air can be compressed and is elastic.
When air is warm, it becomes lighter than cold air. It contracts when cooled and expands when heated.
Air has the ability to retain heat and is nearly impermeable.
It always fills the entire volume and occupies any empty space it can find.
Air is crucial for the process of combustion.
Composition of air: What is air made of? Components of air:
In 1754, Joseph Black conducted an experiment that demonstrated the fact that air is not a simple substance but instead a mixture of gases.
* In relation to dry air (without any water vapor).
** The concentration of water vapor can vary significantly from approximately 0.0001% by volume in the coldest regions of the atmosphere to 5% by volume in hot, humid air masses (in terms of dry air).
*** Water vapor makes up about 0.25% by mass of the entire atmosphere.
There is a possibility of various naturally occurring substances being present in the air in small amounts that can vary locally and seasonally, such as aerosols. These substances include dust composed of different mineral and organic substances (such as sulphur and sulphur compounds: hydrogen sulphide, sulphur dioxide, etc.), pollen and spores, sea spray, and volcanic ash.
In addition, gases or aerosols from various industrial pollutants (such as sulfur, chlorine, and their compounds) may also be found in the air.
Air composition can differ within narrow ranges: in urban areas, the level of carbon dioxide is slightly elevated compared to forests, while in high mountains and at high altitudes, the concentration of oxygen is slightly lower. This is because oxygen molecules are heavier than nitrogen molecules, causing the oxygen concentration to decrease more rapidly with increasing altitude.
Nitrogen is the primary constituent of air, accounting for 78.084% by volume and 75.5% by mass, making it one of the most abundant elements on Earth.
Nitrogen is an essential chemical element for the survival of animals and plants. It plays a crucial role in various organic compounds such as proteins, amino acids, nucleic acids, nucleoproteins, chlorophyll, and hemoglobin. Within living cells, nitrogen atoms make up approximately 2% of the total number of atoms and around 2.5% of the mass (making it the fourth most abundant element after hydrogen, carbon, and oxygen).
In its pure form, nitrogen exists as a colorless, odorless gas composed of two atoms. It exhibits high chemical inertness.
Industrial nitrogen is produced by separating air into its individual components. Approximately three-quarters of the industrial nitrogen is utilized for ammonia synthesis, while the remaining one-quarter serves as an inert medium in various industrial processes. Liquid nitrogen finds applications as a refrigerant.
Oxygen is the second most abundant component in the Earth’s atmosphere, following nitrogen. It makes up approximately 20.9476% of the volume and 23.15% of the mass of air. Nitrogen and oxygen together constitute about 99% of the total atmospheric air.
Throughout the Cambrian period, which began around 540 million years ago, the oxygen levels in the air ranged from 15% to 30% by volume. By the end of the Carboniferous period, approximately 300 million years ago, the oxygen levels reached their peak at around 35% by volume. This high oxygen concentration may have played a role in the evolution of larger insects and amphibians during that time.
Since then, the oxygen levels in the air have gradually decreased and stabilized at their present-day levels.
Furthermore, oxygen is the most abundant element in the Earth’s crust, constituting approximately 47% of its total mass. It is found in over 1500 compounds, primarily silicates.
Oxygen is a highly reactive non-metal. In its pure form, it is a colorless, odorless, and tasteless gas composed of two oxygen atoms (O2).
Oxygen is a vital component of numerous organic substances and is present in all living cells. It plays a crucial role in proteins, fats, carbohydrates, amino acids, nucleic acids, nucleoproteins, chlorophyll, hemoglobin, among others. It accounts for approximately 25% of the total number of atoms in living cells and around 65% of their mass.
The primary function of oxygen in biological systems is to serve as a vital component for respiration in most living organisms. Molecular oxygen plays a crucial role in energy synthesis processes within these organisms.
The transfer of oxygen from the atmosphere to the bloodstream and subsequently to the tissues relies on the disparity in its partial pressure. Hence, it is the partial pressure of oxygen, rather than the percentage of oxygen in the air, that holds biological significance. At sea level, the partial pressure of oxygen measures 160 mm. Once it falls to 140 mm, individuals begin exhibiting initial signs of hypoxia. A reduction in partial pressure to levels between 50-60 mm poses a severe threat to life.
Argon is the third most abundant component of the Earth’s atmosphere, following nitrogen and oxygen. It makes up 0.934% of the volume and 1.292% of the mass of air.
Argon is a colorless, odorless, and tasteless inert gas. It does not react chemically with other substances.
While argon does not have a significant role in biological processes, inhaling argon can be harmful to health as it prevents oxygen from entering the lungs.
Carbon dioxide (CO2) is a colorless gas under normal conditions. It is mostly odorless, although in high concentrations it can have a sour “soda” smell, reminiscent of carbonated water. The concentration of carbon dioxide in the atmosphere is 0.0314% by volume and 0.046% by mass. It is approximately 1.5 times heavier than air.
Carbon dioxide is a gas that easily transfers radiation in the ultraviolet and visible parts of the spectrum. This radiation comes from the Sun and helps to heat the Earth. Additionally, carbon dioxide absorbs infrared radiation emitted by the Earth. It is one of the greenhouse gases that contribute to global warming. Since the beginning of the industrial era, there has been a steady increase in the level of carbon dioxide in the atmosphere.
Living organisms produce carbon dioxide as one of the byproducts of metabolism in their tissue cells. This gas is then carried from the tissues through the venous system and ultimately excreted through the lungs when we exhale. On average, the human body excretes about 1 kg of carbon dioxide per day.
Photosynthesis is a process that involves the participation of carbon dioxide. Therefore, from March to September, the level of CO2 in the atmosphere decreases due to photosynthesis, and then increases from October to February.
While carbon dioxide is not considered toxic, high concentrations of it in the air can be harmful to living organisms that rely on air for respiration. In fact, according to GOST 8050-85, carbon dioxide is categorized as a hazardous substance belonging to the 4th class of danger.
Carbon dioxide acts as a stimulant for the respiratory center. When its concentration in the air reaches 0.5% and above, it leads to an increase in pulmonary ventilation. Small increases in concentration, up to 2-4%, in enclosed spaces can cause drowsiness and weakness in individuals. Concentrations of approximately 7-10% are considered hazardous to health, as they can cause symptoms of suffocation such as headache, dizziness, hearing loss, and loss of consciousness (similar to symptoms of altitude sickness). The onset of these symptoms depends on the concentration and can occur within a few minutes to one hour. Inhaling air with very high concentrations of carbon dioxide, even with a high oxygen content, can result in rapid suffocation due to hypoxia.
While neon does not have a significant biological role, inhaling it can be hazardous to one’s health because it prevents the entry of oxygen into the lungs.
Neon, when mixed with helium in a neon-helium blend, is used for breathing purposes by oceanauts, divers, and individuals who work under high pressures to prevent gas embolism and nitrogen narcosis. The advantage of this mixture is that it generates less body heat due to the lower thermal conductivity of neon compared to helium.
Methane is the simplest hydrocarbon and is a colorless, tasteless, and odorless gas under normal conditions.
The concentration of methane in the air is 0.0002% by volume and 0.000084% by mass. Methane is approximately twice as light as air.
Methane, when combined with air or oxygen, is both flammable and explosive.
Methane is a potent greenhouse gas, surpassing the effects of carbon dioxide. It accounts for 4-9% of the greenhouse effect. If we consider the impact of carbon dioxide on the climate as one unit, then the greenhouse activity of the same volume of methane would be 21-25 units.
Methane falls under the 4th class of hazardous substances according to GOST 12.1.007, but it is considered to be low hazard.
Helium is an inert gas with no color, taste, or odor.
The concentration of helium in the air is 0.000524% by volume and 0.000073% by mass.
Although helium has no biological function, inhaling it can be dangerous to health because it does not provide any oxygen to the lungs.
Krypton is an inert gas with no color, taste, or odor.
The amount of krypton present in the air is 0.000114% by volume and 0.0003% by mass.
Krypton does not serve any biological purpose. However, inhaling krypton can be harmful to health as it prevents oxygen from entering the lungs.
Hydrogen is the lightest element on the periodic table of chemical elements created by D.I. Mendeleev. The concentration of hydrogen in the air is 0.00005% by volume and 0.00008% by mass.
Under standard temperature and pressure, hydrogen is a colorless, odorless, and tasteless, non-toxic diatomic gas with the chemical formula H2. When mixed with air or oxygen, it becomes flammable and explosive.
While oxygen can be found on Earth in both bound and free forms, the majority of hydrogen on our planet exists in the form of compounds. Only a tiny fraction of hydrogen is present in the atmosphere as a pure element.
Hydrogen is an integral part of nearly all organic substances and is present in all living cells, where it makes up approximately 63% of the total number of atoms.
Therefore, the significance of hydrogen in the chemical processes that occur on Earth and within living organisms is nearly equal to that of oxygen.
Hydrogen, as a component of air, does not play a significant biological role. However, xenon, on the other hand, is an inert, one-atom gas that lacks color, taste, and odor.
The concentration of xenon in the air is 0.0000087% by volume and 0.00004% by mass.
Xenon does not serve any biological purpose. However, inhaling xenon can be dangerous to health because it prevents oxygen from reaching the lungs.
Water vapor is one of the components found in the air. Its concentration varies greatly, ranging from about 0.0001% by volume in the coldest parts of the atmosphere to 5% by volume in hot, humid air masses (in terms of dry air). Water vapor makes up about 0.25% by mass of the entire atmosphere.
The amount of water vapor in the air is influenced by factors such as temperature, humidity, time of year, and climate. For example, at a temperature of 0 °C, 1 m³ of air can hold a maximum of 5 g of water, while at a temperature of +10 °C, it can hold up to 10 g.
Water (hydrogen oxide) is a binary inorganic compound with the chemical formula H2O. A molecule of water is made up of two atoms of hydrogen and one atom of oxygen, which are linked together by a covalent bond. At typical temperatures and pressures, water is a clear liquid that lacks color (when the thickness of the layer is thin), scent, or flavor. In its solid form, water is referred to as ice (which can form snow or frost), and in its gaseous state, it is called water vapor.
Characteristics of air:
| Parameter Name | Value |
| Color | colorless |
| Taste | tasteless |
| Odor | odorless |
| Transparency | completely transparent |
| Average molar mass (average mass of one mole of substance), g/mol | 28.98 |
| Density dry air at normal atmospheric pressure (101 325 Pa or 1 atm) and temperature 0 °C , kg/m 3 | 1.292 |
| Density dry air at normal atmospheric pressure (101 325 Pa or 1 atm) and temperature 0 °C , g/cm 3 | 0.001292 |
| Density dry air at normal atmospheric pressure (101 325 Pa or 1 atm) and temperature 20 °C , kg/m 3 | 1.2041 |
| Density dry air at normal atmospheric pressure (101 325 Pa or 1 atm) and temperature 20 °C , g/cm 3 | 0.0012041 |
| Boiling point of air at normal atmospheric pressure, o C | -192 |
| Melting point of air at normal atmospheric pressure, o C | -213 |
| Average specific heat capacity at constant pressure (101 325 Pa or 1 atm.), kJ / (kg-K) | 1.006 |
| Average specific heat capacity at constant volume (at normal atmospheric pressure), kJ/(kg-K) | 0.717 |
| Adiabatic index of air (ratio of heat capacity at constant pressure to heat capacity at constant volume) (at normal atmospheric pressure). | 1.40 |
| Thermal conductivity of air at 0 ℃ and normal atmospheric pressure, W/(m-K) | 0.0243 |
| Sound velocity in air at normal conditions, m/s (km/h) | 331 (1193) |
| Average coefficient of thermal expansion of air in the temperature range 0-100°C (volume change with gradual temperature increase at constant normal atmospheric pressure), 1/K | 3.67×10-3 |
| Coefficient of dynamic viscosity of air at normal conditions and normal atmospheric pressure (dynamic viscosity – internal resistance of molecules to movement inside the substance according to Newton’s law), μPa-sec. | 17.2 |
| Solubility of air in watercm 3 /L | 29.18 |
| Refractive index of air at normal conditions and normal atmospheric pressure (refractive index means the change in the angle of motion of light and any other waves in a substance). | 1.0002926 |
| Refractive index change coefficient (at normal conditions and normal atmospheric pressure), 1/Pa | 2.8×10-9 |
| Average polarizability of a molecule (under normal conditions and normal atmospheric pressure) | 1.7×10-30 |
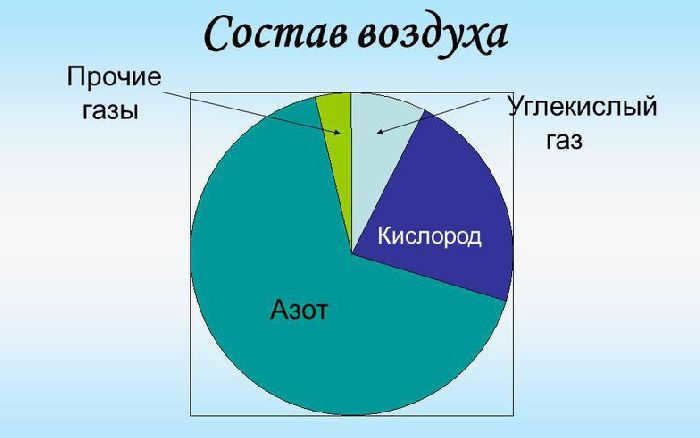
The Earth’s air envelope is known as the atmosphere, consisting of a combination of gases such as nitrogen, oxygen, carbon dioxide, and various other gases. Nitrogen accounts for 78% of the atmosphere, while oxygen makes up 21%, with the remaining 1% comprising other gases.
What are some tips for crafting a high-quality answer?
- Ensure your answer is accurate and valid.
- Provide a detailed and clear response to maximize its usefulness.
- Demonstrate competence by avoiding grammatical, spelling, and punctuation errors, as this enhances understanding.
Navigating is a term that refers to the action of steering or directing a ship or boat. There may be several synonyms to this term.
1. Mariner. The seasoned mariner gazed off into the distance, contemplating the upcoming treacherous voyage;
2. The voyager. The voyager donned weathered trousers, and his shirt was saturated with the scent of the ocean and salt;
3. The seafarer. The experienced sailor was aware that many vessels had already met their demise in this location, as sharp rocks lay hidden beneath the water’s surface;
4. The sea dog. The seasoned sea dog was filled with joy, as he was on the brink of embarking on a lengthy expedition.
The primary components of clean atmospheric air are found in the following proportions (% by volume): nitrogen – 78.03; oxygen – 20.95; ozone and other inert gases like argon, helium, neon, krypton, xenon, radon – 0.94; carbon dioxide – 0.03; water vapor – 0.05. The levels of carbon dioxide in the air are assumed to be equal (% by volume): 0.03 in rural areas and 0.04-0.07 in cities. The amount of water vapor in the air varies depending on its temperature. Ozone is present in forest, mountain, and sea air. Outdoor air becomes polluted by harmful gases and dust emitted from industrial factories.

Module 1: Understanding the Composition of Air
Interestingly enough, Joseph Black’s initial interests lay solely in the humanities, specifically philosophy. If it weren’t for his father’s firm guidance and urgent suggestions to delve into the realms of physics and chemistry, this groundbreaking discovery may never have come to fruition.
Lesson 2: The Composition of Air

To begin with, it should be mentioned that nitrogen constitutes the largest portion of the atmosphere. Nevertheless, the chemical composition of the remaining portion is quite intriguing and varied. In short, here is a rundown of the primary elements.
That being said, let us delve into the functions of these chemical elements.
The volume of air consists of 78% nitrogen and it makes up 75% of its mass. This element is widely present on Earth and also found beyond human-inhabited areas such as Uranus, Neptune, and interstellar space. Having determined the amount of nitrogen in the air, let’s now explore its functions. Nitrogen is crucial for the existence of living organisms as it is a component of proteins, amino acids, nucleic acids, chlorophyll, hemoglobin, and other essential substances.
Typically, nitrogen atoms make up approximately 2% of a living cell, which is why the air contains such a high percentage of nitrogen in terms of volume and mass. Nitrogen is also one of the inert gases obtained from the atmosphere. It is utilized in the production of ammonia, as well as for cooling and various other applications.
2. Oxygen
Oxygen is an essential element for life on Earth. It is a colorless and odorless gas that makes up about 21% of the Earth’s atmosphere. Without oxygen, most living organisms would not be able to survive.
Oxygen is also necessary for the process of respiration, which is how living organisms convert food into energy. When we breathe in, our lungs take in oxygen from the air and deliver it to our cells. The cells then use oxygen to break down glucose and produce ATP, which is the energy currency of the cell.
In addition to its role in respiration, oxygen is also important for many other biological processes. It is needed for the synthesis of proteins, DNA, and other molecules essential for life. Oxygen is also involved in the immune system’s ability to fight off pathogens and in the process of healing wounds.
While oxygen is essential for life, it can also be harmful in high concentrations. Oxygen toxicity can occur when the body is exposed to increased levels of oxygen for a prolonged period of time. This can lead to damage to the lungs and other organs.
Overall, oxygen plays a crucial role in supporting life on Earth. Without it, life as we know it would not be possible.
The question of the oxygen content in the atmosphere is a widely discussed topic. To add an element of surprise, let’s delve into a fascinating fact: oxygen was actually discovered twice – once in 1771 and then again in 1774. However, due to discrepancies in the publication of the discoveries, the credit for the element’s discovery went to the English chemist Joseph Priestley, who isolated oxygen second. As for the actual proportion of oxygen in the air, it fluctuates around 21% by volume and 23% by mass. When combined with nitrogen, these two gases constitute a whopping 99% of the Earth’s atmosphere. Interestingly, despite oxygen being present in a smaller percentage than nitrogen, we have no difficulty breathing. This is because the amount of oxygen in the air is perfectly tailored for normal respiration. In its pure form, oxygen can actually be toxic to the body, causing disruptions in the nervous, respiratory, and circulatory systems. On the other hand, insufficient oxygen levels can also have detrimental effects on health, leading to symptoms of oxygen deprivation. Therefore, the current amount of oxygen in the air is precisely what we need for healthy and complete breathing.
3- Argon
Argon is the third gas found in the earth’s atmosphere. It is characterized by being odorless, colorless, and tasteless. Although no significant biological function has been discovered for this gas, it does have a narcotic effect and is even regarded as a drug. Argon, when extracted from the atmosphere, is utilized in various industries such as medicine, to generate artificial atmospheres, for chemical synthesis, fire suppression, laser production, and more. Furthermore, argon finds application in the field of chemical manufacturing.
4. Carbon dioxide
5. Neon
Neon, the fifth most common chemical element, is utilized in various ways, including disco lights, brightly colored signs, and modern headlights. Humans also inhale this gas, which, like many inert gases, has a narcotic effect on humans at certain pressures. Neon is specifically used in the training of divers and individuals who work at elevated pressures. Additionally, neon-helium mixtures are employed in medicine for respiratory disorders. Neon itself is used for cooling purposes and in the production of signal lights and neon lamps. Despite common belief, neon light is not blue but rather red. Different colors are achieved through the use of lamps containing other gases.
6. Methane
Methane has a long and ancient history, dating back to the primary atmosphere before the existence of humans. It was present in much larger quantities at that time. Today, methane is still emitted from the Earth, although it is not as widely distributed in the atmosphere as it once was. This gas is extracted and used as fuel and raw material in production. While modern research acknowledges the role of methane in human respiration and activity, there is no definitive data on its impact.
7. Helium
When considering the quantity of helium present in the atmosphere, it becomes apparent that this particular gas does not rank among the most vital. In fact, determining the biological significance of helium proves to be quite challenging. Excluding the amusing voice distortion that occurs when inhaling helium from a balloon, helium finds extensive use in various industries. Examples of its applications include metallurgy, the food industry, filling airships and weather balloons, laser technology, nuclear reactors, and more.
We are not discussing the place where Superman was born 🙂 Krypton is a noble gas that has a density three times greater than that of air, chemically unreactive, extracted from the atmosphere, employed in light bulbs, lasers, and still under active investigation. Among the intriguing characteristics of krypton, it should be highlighted that at a pressure of 3.5 atmospheres, it induces a narcotic effect on individuals, and at 6 atmospheres, it develops a strong smell.
9. Hydrogen
Despite its low concentration in air (0.00005% by volume and 0.00008% by mass), hydrogen is actually the most abundant element in the universe. Its rich history, diverse production methods, and wide range of applications make it a fascinating subject to explore. While a comprehensive article could be written on this topic, we will focus on a brief overview of the industries that rely on hydrogen: chemical, fuel, food, aviation, meteorology, and electricity.
10. Xenon
Lesson 3: Air’s Physical Properties

Nowadays, it is possible to determine the physical characteristics of air, just like any other mixture of substances.


Air is an essential mixture of gases that is vital for supporting and sustaining life on our planet. What are the distinct characteristics of air and the specific substances that comprise it?
The Composition of Air
Air is indispensable for the respiration of all living organisms. It is primarily made up of nitrogen, oxygen, argon, carbon dioxide, and various impurities. The exact composition of atmospheric air can vary depending on environmental conditions and geographical location. For instance, in urban areas, the levels of carbon dioxide in the air tend to be higher compared to forested regions due to the abundance of vehicles. At higher altitudes, the concentration of oxygen decreases because nitrogen molecules are lighter than oxygen molecules, resulting in a more rapid decline in oxygen concentration.
In 1754, the Scottish physicist and chemist Joseph Black conducted experimental research that conclusively demonstrated that air is not merely a substance, but rather a precise mixture of gases.

If we consider the makeup of the Earth’s atmosphere in terms of percentage, the primary constituent is nitrogen. Nitrogen makes up 78% of the total volume of the atmosphere. Oxygen, on the other hand, accounts for 20.9% of the air molecule. Nitrogen and oxygen are the two predominant elements in the atmosphere. The remaining substances make up a much smaller percentage, less than 1%. For instance, argon comprises 0.9% of the atmosphere, while carbon dioxide makes up only 0.03%. Additionally, the atmosphere contains trace amounts of impurities such as neon, krypton, methane, helium, hydrogen, and xenon.
The aeroionic composition of the air is of great importance in industrial premises. The presence of negatively charged ions in the air has a positive impact on the human body, providing energy and boosting mood.
Nitrogen, a second-period element, does not have excited states in its ground state, as its atom lacks free orbitals. However, nitrogen can exhibit a valence of not only III but also IV in its ground state by forming covalent bonds through a donor-acceptor mechanism involving the unshared electron pair of nitrogen. The oxidation states that nitrogen can assume vary widely, ranging from -3 to +5.
Nitrogen exists in nature in two forms: as a simple substance, specifically as gas N2, and in a bound state. A nitrogen molecule is formed by two atoms that are strongly bonded together by a triple bond, which has an energy of 940 kJ/mol. At normal temperatures, nitrogen can only interact with lithium. However, if the molecules are activated beforehand through heating, irradiation, or the use of catalysts, nitrogen can react with both metals and nonmetals.
Oxygen
Oxygen is the most prevalent element on Earth: it accounts for 47.3% of the Earth’s crust by mass and 20.95% of the atmosphere by volume. In living organisms, oxygen makes up about 65% of the mass.
In almost all compounds, except for those containing fluorine and peroxides, oxygen has a constant valence of II and an oxidation state of -2. The oxygen atom does not have any excited states because there are no unoccupied orbitals in the second outer shell. Oxygen exists in two allotropic forms as a pure substance: oxygen gas (O2) and ozone gas (O3). The most significant compound containing oxygen is water. Approximately 71% of the Earth’s surface is covered by water, and life would not be possible without it.
Ozone is naturally formed from oxygen in the air during thunderstorms and can also be produced in the laboratory by passing an electrical discharge through oxygen.
Ozone is a more potent oxidizer compared to oxygen. Specifically, it has the ability to oxidize gold and platinum.
In industrial settings, oxygen is typically obtained by the process of liquefying air and subsequently separating nitrogen through the process of vaporization. This separation is possible due to the difference in boiling points, with liquid oxygen having a boiling point of -183 degrees and liquid nitrogen having a boiling point of -196 degrees.