In our elementary school natural science classes, we were taught that air is a combination of gases and is not solely made up of oxygen. However, as time went on, we seemed to forget the exact makeup of air. Today, we invite you to revisit this subject with us and examine the chemical properties and composition of air.
Lesson 1: Understanding the Nature of Air
So, when we talk about atmospheric air, we are referring to a combination of different gases. However, this description is not comprehensive enough. To delve deeper into the topic, let’s explore its historical background. Back in 1754, a Scottish physicist and chemist named Joseph Black stumbled upon an intriguing phenomenon while heating white magnesia. He discovered the liberation of “bound air,” which turned out to be carbon dioxide – a gas that has gained popularity on our blog :). This breakthrough led Mr. Black to another significant revelation – the composition of air, previously believed to be a single substance, is actually a mixture of various components.
Interestingly, Joseph Black’s original interests lied in the humanities, particularly philosophy. If it weren’t for his father’s firm guidance and urgent advice to pursue physics and chemistry, this remarkable discovery may never have come to light.
Joseph Black was indeed the one who paved the way for other scientists, who subsequently began unraveling the composition of the atmosphere, measuring the levels of oxygen and other gases in the air. It was through their collective efforts that the modern definition of air emerged: a mixture of gases that forms the Earth’s atmosphere. The primary purpose of air is to create a suitable environment for breathing and the sustenance of living organisms. This importance led to the creation of the federal law of the Russian Federation, known as “On the protection of atmospheric air.” Furthermore, the atmosphere serves as a source of inert gases, which can be extracted through the process of liquefaction. So, what exactly are the gases that make up the composition of air?
Lesson 2: The Composition of Air

It should be noted from the start that the air is predominantly composed of nitrogen, but the remaining portion exhibits a fascinating and varied chemical composition. To summarize, the fundamental elements in this composition are as follows:
Nevertheless, we will provide a brief elucidation of the roles these chemical elements play.
1. Nitrogen
Air contains nitrogen, which makes up 78% by volume and 75% by mass. This element is abundant on Earth and can also be found on Uranus, Neptune, and in interstellar space. Nitrogen plays an important role in the existence of living organisms as it is a component of proteins, amino acids, nucleic acids, chlorophyll, hemoglobin, and more.
2. Oxygen
Oxygen is an essential element for human survival. It is necessary for the process of respiration, where it is used by the body to convert nutrients into energy. Without oxygen, our cells would not be able to function properly, and our organs would not receive the oxygen they need to perform their functions. Oxygen is also important for the maintenance of our immune system and for the detoxification of harmful substances in the body. In addition to its role in human health, oxygen is also crucial for the survival of other organisms, such as plants and animals.
The oxygen concentration in the atmosphere is a subject that has piqued the curiosity of many. Let’s delve into an intriguing tidbit: oxygen was actually discovered twice, once in 1771 and again in 1774. However, due to discrepancies in the publication of the discoveries, the credit for the discovery of this element goes to the English chemist Joseph Priestley, who isolated oxygen second. The proportion of oxygen in the atmosphere hovers around 21% by volume and 23% by mass. Alongside nitrogen, these two gases make up a staggering 99% of the Earth’s atmosphere. Interestingly, despite oxygen being present in a lower percentage than nitrogen, we have no difficulty breathing. This is because the amount of oxygen in the atmosphere is perfectly calibrated for normal respiration. In its pure form, oxygen can be toxic to the body, causing disruptions in the nervous, respiratory, and circulatory systems. On the other hand, a deficiency in oxygen can also have negative effects on health, leading to symptoms of oxygen deprivation. Therefore, the quantity of oxygen in the atmosphere is precisely what is necessary for healthy, unrestricted breathing.
Argon is the third element in the air; it lacks smell, color, and taste. While no significant biological function has been attributed to this gas, it does possess narcotic properties and is even classified as a drug. Argon, obtained from the atmosphere, finds applications in various industries and medical fields, such as the creation of artificial atmospheres, chemical synthesis, fire suppression, and the development of lasers, among others.
While Venus and Mars have a significant amount of carbon dioxide in their atmospheres, the percentage of carbon dioxide in Earth’s air is comparatively lower. However, a substantial amount of carbon dioxide is present in the ocean, constantly replenished by various organisms and industrial emissions. Carbon dioxide also plays important roles in human life, such as in firefighting and as a gas and food additive (known as E290) in the food industry, where it acts as a preservative and leavening agent. In its solid form, carbon dioxide is widely recognized as “dry ice,” a popular refrigerant.
Neon, the fifth most abundant element in the universe, is the same mysterious light that illuminates disco lights, brightly colored signs, and modern headlights. It is interesting to note that humans also inhale neon, as it is a common chemical element. Similar to other inert gases, neon can have a narcotic effect on humans when exposed to certain pressures. In fact, divers and individuals who work at elevated pressures often use neon gas for training purposes.
In addition to its use in diving, neon-helium mixtures are also utilized in medicine to treat respiratory disorders. Furthermore, neon itself is employed for cooling purposes and plays a crucial role in the production of signal lights and neon lamps. Despite popular belief, neon light is not blue but rather red. It is worth mentioning that lamps containing other gases are responsible for producing all the other colors.
Methane and air have a long and ancient history: in the original atmosphere, prior to the emergence of mankind, methane existed in significantly greater quantities. Although currently extracted and utilized as a fuel and raw material in various industries, this gas is not as prevalent in the atmosphere as it once was, but it continues to be emitted from the Earth. Recent studies are shedding light on the role of methane in human respiration and activity, but there is no definitive data on this matter.
7. Helium
When considering the quantity of helium present in the atmosphere, it becomes apparent that this particular gas does not hold a particularly high level of importance. In fact, it is quite challenging to ascertain the biological relevance of this gas. Aside from the amusing voice alteration that occurs when inhaling helium from a balloon, helium finds widespread usage in various industries such as metallurgy and the food industry. Additionally, it is utilized for filling airships and weather balloons, as well as in the fields of lasers and nuclear reactors.
Let’s discuss something different, not the place where Superman was born 🙂 Krypton is actually a noble gas that is three times denser than air. It is chemically unreactive and can be extracted from the atmosphere. Krypton has various uses, such as in light bulbs and lasers. It is also an area of active research. One fascinating aspect of krypton is that it can have a narcotic effect on humans when exposed to a pressure of 3.5 atmospheres. Additionally, at 6 atmospheres, it develops a strong and pungent smell.
Hydrogen comprises 0.00005% of the air’s volume and 0.00008% of its mass, yet it remains the most abundant element in the entire Universe. The history, production, and application of hydrogen could easily fill an entire article, but for now, let’s focus on a concise compilation of industries where hydrogen plays a crucial role: chemical, fuel, food processing, aviation, meteorology, and electric power.
The final component found in air was initially regarded as a mere impurity to krypton. It is called xenon, which translates to “foreigner,” and its presence is quite scarce both on Earth and beyond, making it quite expensive. Nowadays, xenon is indispensable in various fields: it is used in the production of robust and pulsating light sources, as well as in medical diagnostics and anesthesia. It is also utilized in spacecraft engines and serves as rocket fuel. Furthermore, inhaling xenon can significantly lower one’s voice (in contrast to the effect of helium), and it has recently been included in the list of banned substances due to its performance-enhancing properties.

Nowadays, the physical characteristics of air can be determined, just like with any combination of substances.

Regardless of the air’s composition, it is important to make an effort to eliminate “chemical additives” originating from industrial activities and, if possible, only inhale clean, fresh air in urban areas, which can be heated by a high-quality air purifier, or in natural environments. Air, in any case, is a crucial yet enigmatic cocktail for non-experts that is vital for life. Throughout history, humanity has often romanticized the concept of air, praising its ability to transcend boundaries and its lightness, dubbing it a symbol of freedom.
Breathe in pure, unpolluted air and maintain good health!
The atmosphere consists of various chemical elements that sustain life as we know it, even in conditions of outer space that would be lethal to it.
Nitrogen, argon, and oxygen are the three most vital gases for supporting life, of which there are many more.
What is the nature of the atmosphere?
The atmosphere comprises the gases that form the outermost and least dense layer of the planet. Its composition varies with altitude due to the different pressures exerted on them. In simpler terms, it is commonly referred to as air and extends up to 11 kilometers in altitude, starting from the surface of the ocean.
At this level, the primary gases present are nitrogen, which accounts for 78%, followed by oxygen at 21%, and then argon at 0.93%. Further down the scale, we find carbon dioxide and water vapor.
Throughout time, the Earth has undergone a series of changes brought about by different organisms, including humans. Humans, for instance, inhale oxygen and exhale carbon dioxide, which is then utilized by plants. This reciprocal relationship between humans and plants is just one example of how the Earth’s ecosystem has evolved. Additionally, the hydrosphere plays a crucial role in regulating temperatures, mitigating extreme conditions that can occur during both day and night.

List of the main atmospheric gases
As previously mentioned, the Earth’s atmosphere consists of a variety of gases, each occupying a different amount of space. Below is a breakdown of these gases in order of abundance.
Nitrogen
Nitrogen makes up approximately 78% of the entire atmosphere, making it the most abundant gas on our planet. It is a chemical element represented by the letter N, with an atomic weight of 14.01 and an atomic number of 7.
Oxygen, which comprises 28% of the atmosphere, is the second most abundant gas. It has an atomic number of 8, which is higher than that of nitrogen, and is represented by the letter O. Oxygen is essential for life on Earth, serving as a vital component for respiration. Additionally, it acts as a powerful antioxidant and possesses the second highest electronegativity among all elements.
It constitutes 0.93% of the total air volume, deriving its name from the ancient Greeks, who referred to it as “.” due to its lack of reactivity with other elements. With an atomic number of 18, this gas is denoted by a series of letters.
These three elements are the primary components of the atmosphere, while others such as carbon dioxide (CO2) account for 0.4%, neon for 0.0018%, helium for 0.00052%, methane for 0.00017%, krypton for 0.0011%, and hydrogen for 0.00005%. The remaining gases, such as nitrous oxide and carbon monoxide, are present in negligible amounts.
What is the most prevalent gas in the Earth’s atmosphere?

As nitrogen is the most abundant gas in the Earth’s atmosphere, it is essential for supporting life as it comprises the majority of the air that all living organisms breathe. This allows it to penetrate deeper in comparison.
Origin
The term “Nitrium” comes from the Latin language, meaning “generating genes”. It was coined by Daniel Rutherford, a physician and chemist, who conducted an experiment in which he absorbed oxygen and carbon dioxide, leaving nitrogen as a residual element. This discovery took place in 1772, although some medieval alchemists had used nitrogen for certain experiments, as mentioned in their writings.
Nitrogen can be obtained through liquefaction and distillation, and it should be noted that there is an almost limitless supply of this element in the atmosphere.
The production of nitrogen, vital for plant growth and development, is carried out by bacteria through a complex process. These bacteria absorb nitrogen and then become a source of food for herbivorous animals. When these animals excrete waste, the bacteria convert the components back into pure nitrogen.
Nitrogen plays a crucial role in the production of ammonia, which has a wide range of everyday applications and is of great commercial importance. Additionally, methane gas is produced when carbon dioxide and hydrogen react with water vapor.
One of the main uses of methane is in refrigeration systems, which are essential for operating refrigerators, air conditioners in homes and cars. It is widely utilized in residential settings and should be handled with caution due to its extremely low temperature, which can cause severe skin damage upon direct contact.
Ammonia is a key ingredient in numerous commercial products that are vital for various human activities. These include the production of plastic, the creation of specialized livestock feed, the manufacturing of fertilizers, and many others.
Due to the excessive consumption of nitrogen in various forms, including consumer goods and the use of plastic to produce fertilizers for growing crops, it is indirectly ingested.
Here are some potential effects:
- It can lead to vitamin A deficiency.
- It may impair oxygen transportation in the blood.
- It can accelerate the production of nitrosamine, a known carcinogen.
- It is associated with thyroid dysfunction.
The information presented in this article adheres to our editorial guidelines. Please click here to report any inaccuracies.
Write a comment, share your thoughts
Feel free to comment No need to reply
Luisa Fernanda commented
Hello, this website is amazing, it’s the best one I’ve ever come across. I just wanted to let you know that you should continue using it more. Don’t lose motivation. Cheers!
The most abundant gas on Earth is nitrogen, which makes up around 80% of the Earth’s atmosphere.
This element was discovered and identified as a distinct substance during the initial studies of the air.

In 1772, Carl Wilhelm Scheele, a chemist from Sweden, conducted an experiment that proved air to be a combination of two gases. He referred to one of these gases as “fire air” because it supported combustion, while the other gas, which remained after the “fire air” was used up, was called “dirty air” (nitrogen).
Around the same period, other scientists also identified nitrogen. Scottish botanist Daniel Rutherford, who was the first to publish his findings, British chemist Henry Cavendish, and British clergyman and scientist Joseph Priestley all recognized the presence of nitrogen. Furthermore, Priestley and Scheele were both credited with the discovery of oxygen (Sanderson, 2017).
The composition of the atmosphere consists of a combination of various gases in different quantities. The gases that remain constant and do not fluctuate from day to day include nitrogen, oxygen, and argon.
Nitrogen accounts for approximately 78% of the atmosphere, oxygen makes up about 21%, and argon makes up approximately 0.9%. There are also waste gases such as carbon dioxide, nitrogen oxides, methane, and ozone, which collectively make up around 0.1% of the atmosphere (NC Estate University, 2013).
Thus, it can be inferred that nitrogen and oxygen make up roughly 99% of the gases present in the atmosphere.
Other gases, such as carbon dioxide, water vapor, and noble gases like argon, are present in much smaller proportions (BBC, 2014).
Water vapor is the only gas whose concentration varies, ranging from 0 to 4% of the atmosphere depending on location and time of day.
Greenhouse gases, with varying percentages on a daily, seasonal, and yearly basis, possess physical and chemical attributes that enable them to engage with solar radiation and infrared radiation (heat) emitted by the Earth in order to influence the energy equilibrium of the planet….
This is precisely why scientists are closely monitoring the increase in greenhouse gases, such as carbon dioxide and methane, as even small quantities can have a significant impact on both the global energy balance and long-term temperature (NASA, SF).
Nitrogen plays a vital role in supporting life on our planet as it is a fundamental component of proteins and is present in all living organisms….
Organic materials, food, fertilizers, explosives, and toxins contain nitrogen compounds.
While nitrogen is essential for life, excessive amounts can also have harmful effects on the environment.
Derived from the Greek word nitron, meaning “native soda,” and the word gene, meaning “to form,” nitrogen is the fifth most abundant element in the universe….
As previously mentioned, nitrogen gas makes up 78 percent of Earth’s atmosphere, according to the Los Alamos National Laboratory in California, USA. In contrast, nitrogen accounts for only 2.6 percent of the atmosphere on Mars.
The nitrogen molecule has a unique structure with a triple bond, making it highly resistant to breaking and giving it the characteristics of an inert gas.
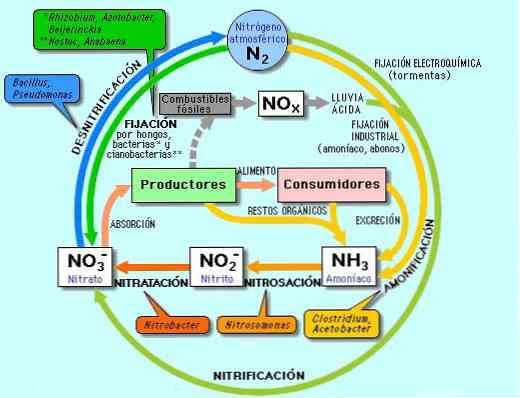
Nitrogen, similar to water and carbon, is a renewable resource found in nature that is replenished through the nitrogen cycle.
The nitrogen cycle, which involves the conversion of atmospheric nitrogen into various organic compounds, is a crucial natural process for sustaining life forms.
In this cycle, bacteria in the soil process or “fix” atmospheric nitrogen into ammonia, which is essential for plant growth.
Other bacteria then convert ammonia into amino acids and proteins, which are consumed by animals when they eat the plants.
Nitrogen compounds are eventually returned to the soil through animal waste, and bacteria convert the remaining nitrogen into nitrogen gas, which is released back into the atmosphere.
People utilize nitrogen in fertilizers to expedite the growth of crops.
However, the excessive usage of these fertilizers in agriculture has had disastrous consequences on the environment and human well-being by contributing to the contamination of ground and surface water….
As stated by the U.S. Environmental Protection Agency (EPA), the pollution of air and water with excess nitrogen and phosphorus is one of the most prevalent, expensive, and intricate environmental issues (Blaszczak-Boxe, 2014).
Nitrogen compounds play a significant role in the creation of ground level ozone. Furthermore, nitrogen compounds in the atmosphere contribute to the generation of acid rain, which not only causes respiratory problems but also has detrimental effects on ecosystems (Oblack, 2016).
- (2014). Earth’s atmosphere. Retrieved from bbc.co.uk.
- Blaszczak-Boxe, A. (2014, December 22). Facts about nitrogen. Retrieved from livescience.com.
- (S.F.). Atmospheric composition Retrieved from science.nasa.gov.
- NC Estate University. (2013, August 9). Atmospheric composition. Retrieved from ncsu.edu.
- Oblak, R. (2016, February 3). Nitrogen – gases in the atmosphere. Retrieved from thought.
- Royal Society of Chemistry. (2017). Nitrogen. Retrieved from rsc.org.
- Sanderson, R. T. (2017, February 12). Nitrogen (N). Retrieved from britannica.com.
A) Oxygen ; b) Nitrogen ; c) Hydrogen ; d) Carbon dioxide.

Nitrogen is the primary component of the earth’s atmosphere : ).

Which is more abundant in the atmosphere: oxygen or carbon dioxide?
Which of the two is found in greater quantities in the atmosphere: oxygen or carbon dioxide?

Provide an analysis of the utilization of oxygen and carbon dioxide
Submit a comprehensive report detailing the various ways in which oxygen and carbon dioxide are utilized.
Identify the gas that has a lower concentration in the atmosphere compared to a) carbon dioxide b) oxygen c) nitrogen d) hydrogen e) argon
Specify the gas that is found in lesser quantities in the air compared to a) carbon dioxide b) oxygen c) nitrogen d) hydrogen e) argon

Calculate the ratio of nitrogen to oxygen in the atmosphere and the ratio of oxygen to carbon dioxide.
Determine how many times more nitrogen there is in the atmosphere compared to oxygen, as well as how many times more oxygen there is in the atmosphere compared to carbon dioxide.
Can you provide assistance?
What is the term for the northernmost point on the Earth?
D) North Pole
Which gas is the most abundant in the Earth’s atmosphere?
D) Nitrogen
Which city does the Prime Meridian pass through?
A) Greenwich
Which sea is not real?

Which is correct: there is more carbon dioxide than nitrogen, or there is more carbon dioxide than oxygen in the composition of atmospheric air?
Which statement is accurate: 1) there is more carbon dioxide than nitrogen, or 2) there is more carbon dioxide than oxygen in the composition of atmospheric air?

What is the significance of atmospheric gases in sustaining life on Earth?
The importance of atmospheric gases in supporting life on Earth.

Which gas makes up the largest proportion in Earth’s atmosphere – 1) oxygen 2) nitrogen 3) hydrogen 4) carbon dioxide?
When it comes to the gas composition of Earth’s atmosphere, the largest fraction is made up of which gas – 1) oxygen 2) nitrogen 3) hydrogen 4) carbon dioxide.

Is the increase in the amount of nitrogen, oxygen, carbon dioxide, or hydrogen in the atmosphere thought to be the cause of global warming?
The cause of global warming is believed to be an increase in the amount of nitrogen, oxygen, carbon dioxide, or hydrogen in the atmosphere.

Which gas among the following is considered a greenhouse gas?
Which gas among the options provided is classified as a greenhouse gas?
If you are looking for an answer to the query “What gas is most prevalent in the Earth’s atmosphere?” in relation to the academic level of students in grades 5 to 9, you have come to the right page. In the Geography category, you will also find responses to similar questions on the relevant subject by utilizing the automatic intelligent search feature. If, after reviewing all the answer choices, you still have doubts or if the information you have obtained does not completely address your query, you can create your own question using the button at the top of the page or engage in a discussion with other visitors on this page.

This region is characterized by four distinct climatic belts that extend along the latitudes. The northern Indian Ocean experiences a monsoon climate with frequent cyclones that move towards the coast due to the influence of the Asian continent. You.

The African continent is home to various ethnic groups, with the Negroid race being the most prevalent. One particular country where this race is prominent is South Africa, which was once a European colony.

It’s relatively simple, here you are.


The Nelson River is a Canadian river that spans a length of 640 kilometers. It originates from Lake Winnipeg and flows into Hudson Bay. The river serves as a drainage system for the Bow-Saskatchewan-Nelson lake-river system, covering an area of 1072 thousand km2. Known for its rapids, the Nelson River experiences an average water flow of 23 cubic meters per second at its mouth.

There is the postscript. It can be located online))).

The indicators of stable, precipitation-free weather are as follows: – in clear weather, the temperature drops gradually, the atmospheric pressure slowly rises, and small cumulus clouds with irregular shapes begin to form. The wind blows from the north-east or east direction; – temp.


14 A 15 B 16 A 17 C 18 B 19 D 20 B 21 Alice 22 Brummie 23 Hawaii 24 dog 25 COP.
© 2000-2023. When using materials in whole or in part, please provide a reference. 16+
The website is secured by reCAPTCHA technology, which is subject to Google’s Privacy Policy and Terms of Use.
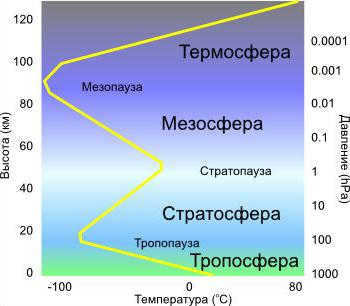
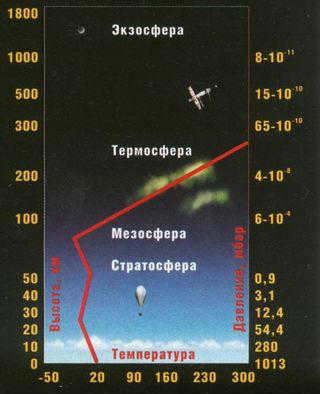
The structure and composition of the Earth’s atmosphere have not always remained constant during different periods of our planet’s development. Currently, this element is divided into several main layers, with a total “thickness” of 1.5-2.0 thousand kilometers, including:
- Troposphere.
- Tropopause.
- Stratosphere.
- Stratopause.
- Mesosphere and mesopause.
- Thermosphere.
- Exosphere.

The primary components of the atmosphere
The troposphere is a region where significant upward and downward movements occur, playing a crucial role in the formation of weather patterns, sedimentary phenomena, and overall climate conditions. It spans approximately 7-8 kilometers above the Earth’s surface, with the exception of polar regions where it extends up to 15 km. Within the troposphere, there is a gradual decrease in temperature, decreasing by approximately 6.4°C for every kilometer of altitude. However, this value may vary depending on different latitudes and seasons.
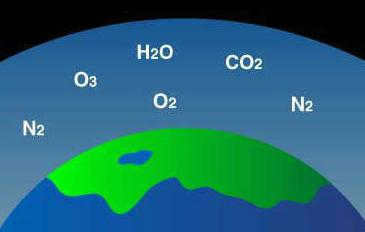
The composition of the Earth’s atmosphere in this layer comprises the following elements and their respective percentages:
– Oxygen – accounting for nearly 21 percent;
– Argon – constituting approximately one percent;
– Carbon dioxide – making up less than 0.05 percent.
Homogeneous Mixture Extends to an Altitude of 90 Kilometers
Furthermore, this region contains various substances such as dust, water droplets, water vapor, combustion byproducts, ice crystals, sea salts, and numerous aerosol particles. This uniform composition is observed throughout the Earth’s atmosphere up to an altitude of approximately ninety kilometers, meaning that the air has a similar chemical makeup not only in the troposphere but also in the higher layers. However, these upper layers possess different physical properties. The region with a consistent chemical composition is known as the homosphere.
Distinctive characteristics of various atmospheric layers in terms of their physical properties
The Earth’s atmosphere contains an essential component called ozone, which is present in relatively small quantities near the surface (less than one millionth of a percent). Ozone is formed in the upper regions of the atmosphere through the interaction of atomic oxygen with sunlight. The highest concentration of ozone is found at an altitude of approximately 25 kilometers, and the entire “ozone shield” is located at different heights depending on the geographic location – ranging from 7-8 kilometers at the poles to 18 kilometers at the equator, with a total extension of up to fifty kilometers above the Earth’s surface.
The air composition of Earth’s atmosphere plays a crucial role in the preservation of life as it effectively restricts solar radiation from reaching the surface and the living organisms inhabiting it. Various chemical elements and compositions work together to achieve this. For instance, water vapor molecules efficiently absorb almost all types of infrared radiation, except for wavelengths between 8 and 13 microns. On the other hand, ozone absorbs ultraviolet radiation up to a wavelength of 3100 A. Without its thin layer (averaging only 3 mm when placed on the planet’s surface), only waters deeper than 10 meters and underground caves, which are shielded from solar radiation, can support life.
The stratopause, located between the stratosphere and the mesosphere, is a unique layer in the atmosphere. It is situated at roughly the same height as the ozone maxima and maintains a relatively comfortable temperature of around 0°C for humans. Moving above the stratopause, we enter the mesosphere, which spans from an altitude of 50 km to 80-90 km. In this region, temperatures once again decrease as we move further away from the Earth’s surface, reaching as low as minus 70-80 °C. The mesosphere is known for its ability to completely incinerate meteors.
Temperature Rise in the Thermosphere: 2000 K and Beyond!
The unique chemical composition of the Earth’s atmosphere within the thermosphere (which begins after the mesopause, at heights ranging from approximately 85-90 to 800 km) gives rise to the fascinating phenomenon of gradual heating of extremely sparse “air” layers under the influence of solar radiation. Within this region of the planet’s atmospheric “blanket,” temperatures ranging from 200 to 2000 K are observed, resulting from the ionization of oxygen (with atomic oxygen present above 300 km) as well as the recombination of oxygen atoms into molecules, which releases significant amounts of heat. It is within the thermosphere that the mesmerizing aurora borealis originates.
How did the Earth’s atmosphere come into existence?
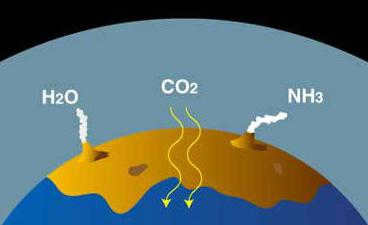
However, it should be noted that the current composition of the planet’s atmosphere did not always exist. There are three main theories regarding the origin of this essential element. The first hypothesis proposes that the atmosphere was acquired during the accretion process from the protoplanetary cloud. Nevertheless, this theory has faced significant criticism as it suggests that the initial atmosphere would have been destroyed by the solar “wind” emitted by the sun in our planetary system. Additionally, it is believed that volatile elements could not have been retained in the formation zone of Earth-like planets due to high temperatures.

Experiment conducted at the Institute for Information Transmission Problems of the Russian Academy of Sciences
Modern humans would not have been able to survive in the Earth’s primary atmosphere without breathing apparatus, as it did not contain enough oxygen. According to scientists, significant amounts of oxygen appeared around one and a half billion years ago, thanks to the evolution of photosynthesis in blue-green algae and other types of algae, which are some of the oldest organisms on our planet.