
In Table 1, you can find the concentration of dry gases along with water vapor, aerosols, radioactive substances, and ions, which are essential components of the lower layers of the atmosphere. These layers play a crucial role in sustaining human life.
Table 1. Breakdown of dry air near the Earth’s surface, %
The composition of the atmosphere is primarily made up of nitrogen, oxygen, and argon, with other gases accounting for only approximately two percent.
The abundance of nitrogen in the air can be attributed to its inertness, allowing it to be preserved in greater quantities compared to other gases during the formation of the atmosphere. The significant presence of oxygen in the Earth’s atmosphere is a result of the photosynthetic process carried out by plants, where oxygen is released and carbon dioxide is consumed.
In addition to these gases, the lower atmosphere also contains varying amounts of ammonia, chlorine and fluorine compounds, radon, and other gases. There are even super-ecotoxicants that were previously unknown to mankind.
Carbon dioxide is released into the atmosphere through various processes such as volcanic eruptions, putrefaction and decomposition of organic matter, respiration of animals and plants, and fuel combustion in industry and transportation. Its concentration in the atmosphere is 0.033%. However, plants play a crucial role in consuming carbon dioxide and producing oxygen. The Siberian taiga and the Amazon jungle are considered the Earth’s main “lungs,” with the taiga being more efficient in producing oxygen due to its lower usage of it for decomposing organic residues. Carbon dioxide is a major contributor to the greenhouse effect as it absorbs and emits long-wave radiant energy. The concentration of carbon dioxide in the air varies depending on factors such as latitude, local conditions, time of day, and time of year. Generally, it is lower at high latitudes compared to temperate latitudes, lower over the ocean compared to land, and lower during daytime hours compared to nighttime. The ocean acts as the main regulator of carbon dioxide concentration, containing about 100 times more carbon dioxide than the atmosphere. This is due to the high solubility of CO2 in water compared to other atmospheric gases. A dynamic equilibrium is established through the exchange of carbon dioxide between air and water. The solubility of gases in water is influenced by temperature, with cold seas and oceans in temperate and high latitudes absorbing approximately the same amount of CO2 as is released into the atmosphere by the tropical oceans. In the biosphere, photosynthesis balances the release of carbon dioxide during respiration of living organisms and decomposition of organic matter.
Unfortunately, sulphur oxide (SO2) and nitrogen oxides (commonly known as NOx) are also widely present in the atmosphere, making them prevalent pollutants.
Water vapor is present in the lower atmosphere and makes up between 0.1% and 4% of the atmosphere’s volume. The amount of water vapor in the atmosphere is influenced by air currents and the characteristics of the Earth’s surface. Water vapor is introduced into the atmosphere through the evaporation of moisture from bodies of water, land, vegetation, and the Earth’s snow and ice cover. It is also released through the respiration of living organisms, volcanic eruptions, the generation of heat and electricity, and certain industrial processes. Once in the atmosphere, water vapor mixes with gases and is carried by the wind over long distances. However, its concentration decreases significantly as altitude increases. Water vapor in the atmosphere condenses to form clouds, which can then lead to precipitation.
Aerosols refer to solid particles and droplets that are suspended in the atmosphere. Typically, these particles are small in size, measuring less than 1 micron. When the particles are larger, they are referred to as dust. It is important to note that even the purest air contains aerosols. Sources of aerosols can be categorized into two groups: natural (such as sea salt evaporation, soil dusting, volcanic activity, and forest fires) and anthropogenic (which include emissions from industries, transportation, and soil erosion). These aerosols have an impact on both climate and biota, referring to all life on Earth. The amount of aerosols present in the atmosphere varies depending on location and time. Some aerosols act as nuclei for the condensation of water vapor. Examples of aerosols include water droplets and ice crystals, fine dust, soot and ash from fires and burning forests and peat bogs, soil, space, and volcanic dust, as well as plant pollen. During the upward movement of air, aerosols can rise to high altitudes, and they can also be carried for long distances by horizontal air flows, such as wind. As altitude increases, the concentration of aerosols decreases significantly.
Smog, a combination of smoke and fog, is classified as an aerosol. Air currents can transport smog up to 100-200 km away from its original source of pollution.
In addition, the atmosphere contains radioactive gases, such as radon, which are challenging to measure accurately. Suspended in the air are solid radioactive materials like strontium 90, uranium 239, and iodine 131. These radioactive substances enter the atmosphere and can persist for decades due to their long half-lives, carried by air currents.
Electrically charged molecules with an electric potential are constantly being generated in the atmosphere, and these molecules are referred to as ions. Ions can be categorized into two groups: light ions and heavy ions. Light ions have the ability to settle on suspended aerosols in the atmosphere, resulting in the formation of larger and heavier ions with significantly greater masses than the light ions. In the lower atmosphere, there are several hundreds of light ions and several hundreds to tens of thousands of heavy ions present in every cubic centimeter of air. As altitude increases, there is a stratification of gases based on density, with the density increasing at higher altitudes. However, up to an altitude of 100 km, gases in the atmosphere are mixed and do not stratify based on density. Gaseous impurities, excluding nitrogen, oxygen, and argon, as well as acidic gases, ozone, and ammonia, are attracted to the Earth’s surface due to their high molecular weight. Above an altitude of 100 km, the tendency towards equilibrium becomes more dominant than mixing, resulting in a faster decrease in the concentration of heavier gases compared to lighter gases. For example, the percentage of argon, which is heavier than nitrogen and oxygen, decreases with increasing altitude. Oxygen molecules at an altitude of approximately 200 km undergo decomposition into charged atoms. In the high atmosphere, nitrogen is also partly present in its atomic state.
The majority of the Earth’s atmosphere is found in a relatively narrow layer near its surface. Approximately half of the atmosphere’s total mass is concentrated in the troposphere, which extends up to approximately 5.5 km in height. Around 75% of the atmosphere’s mass is contained within a height of approximately 11 km, while 95% is found within a height of 20 km.
Human activity over the past century has had a negative impact on the composition of the atmosphere. From the mid-1800s to 1975, the overall carbon dioxide levels increased by 12-15%. The concentration of CO2 is even higher in industrial centers. In megacities, the gas can reach levels of 0.1-0.2% while oxygen levels decrease. Carbon dioxide is the primary contributor to the greenhouse effect. The main sources of CO2, as well as NOx and SO2, in large cities are motor vehicles (accounting for up to 80% of total pollutants), thermal power plants, and other industrial processes involving fuel combustion. Ecologists argue that the relative harmfulness of these three gases is in a ratio of 20:12:1, with nitrogen oxides being the most aggressive, followed by sulfur oxide, and then carbon dioxide. Nitrogen oxides actively contribute to the formation and expansion of ozone holes (alongside freons, NOx depletes the ozone layer), and together with CO2 contribute to the greenhouse effect. When dissolved in water droplets of clouds, nitrogen oxides form weak nitric and nitrous acids, leading to acid rain when they fall to the ground. Sulfur oxide (sulfur dioxide) is released into the atmosphere through the burning of solid or liquid fuels with a high sulfur content, as well as from chemical and metallurgical plants. The use of high-sulfur fuel oil in power generation and diesel fuel in vehicles pose particular risks to urban atmospheres. Currently, many large cities are transitioning to liquid fuels with reduced sulfur content or switching to natural gas combustion.
Sulfur dioxide, carbon monoxide (CO), and nitrogen dioxide have a long lifespan in the atmosphere. The levels of these gases in the atmosphere are significantly higher than the background levels: SO2 – 50-300 times; CO – 80-1250 times; NOx – up to 25 times.
The release of chlorofluorocarbon hydrocarbons, specifically freons, into the atmosphere poses a serious threat to the Earth’s climate. They are commonly used as refrigerants in refrigeration production and have a long lifespan (over a year) as well as the ability to deplete the ozone layer. The most concentrated gases of this kind are methyl chloride, dichlorodifluoromethane, and fluorotrichloromethane.
Methane (CH4) is a key constituent of the atmosphere and plays a significant role in the development of the greenhouse effect. There has been a consistent rise in the concentration of methane in the air, with a 10% increase over the past two decades. The levels of methane reach their peak during spring and fall, while they are at their lowest during winter and summer.
In the previous century, humanity was introduced to previously unfamiliar gaseous substances known as xenobiotics. These substances, also referred to as super ecotoxicants due to their highly dangerous effects on humans, decompose very slowly over long periods of time under natural processes. The primary medium through which they are distributed is the air environment. Notable examples of super ecotoxicants include dioxins, benz-α-pyrene, DDT, and polychlorinated biphenyls. These substances have very low maximum allowable concentrations (MAC). The combustion of polyethylene in oxygen-deficient conditions, as well as the operation of incinerators and the burning of household garbage in landfills, are the main sources of super ecotoxicant formation.
Lower layers of the atmosphere are characterized by radioactive gases, with radon being the most prevalent. These gases are often released through geological faults and garbage disposal sites.
Every year, alongside gases, industrial facilities and transportation emit massive quantities of soot, slag, dust, coal tar, organic acids, and hydrocarbons.
The lower layers of the atmosphere contain the highest concentration of aerosols.
Photochemical smog is the result of atmospheric pollution caused by a combination of harmful gases, vapors, and aerosols. This smog has detrimental effects on humans, animals, and plants. In large cities, local air pollution has reached the level of an ecological disaster.
The primary ecological requirement for air quality is the following standard: the total ratio of actual concentrations of harmful substances to their Maximum Permissible Concentration (MPC) should be less than one.
In cities, the primary methods of air pollution control are increasing green spaces and transitioning industrial facilities to natural gas combustion. Each enterprise must undergo a mandatory environmental impact assessment.
Nature constantly undergoes the processes of air purification. However, the impact of human activities on the environment has overwhelmed nature’s ability to cope, resulting in a worldwide rise in the concentration of certain gases in the atmosphere. This increase has led to adverse effects on the climate, human health, biodiversity, and economy. The combustion of fuel depletes significant amounts of oxygen. If the current trend of increasing fuel consumption persists, experts forecast that approximately 1.0% of the available oxygen in the atmosphere will be depleted within the next 50 years.
Ozone is a molecule composed of three oxygen atoms. It is produced in the Earth’s atmosphere at an elevation ranging from 15 to 70 kilometers due to thunderstorm discharges and the oxidation of organic substances. In higher atmospheric layers, ozone is formed under the influence of the Sun’s ultraviolet rays. Ozone plays a crucial role in shielding all living organisms on our planet from harmful ultraviolet radiation. The existence of life as we know it became possible once the concentration of oxygen surpassed 1% and ozone could form a protective layer in the atmosphere. If we were to gather all the ozone at regular atmospheric pressure, the thickness of the ozone layer would only be 3 millimeters. Nevertheless, even this small quantity is sufficient to absorb 3% of solar radiation and elevate the temperature of the atmosphere at an altitude ranging from 30 to 55 kilometers.
The impact of human activity on the environment is currently resulting in a significant reduction in ozone levels and the formation of ozone holes above the Earth. Meteorological studies have shown that an ozone hole larger than the size of the entire continent has already formed over Antarctica, with similar observations in Tasmania and Tierra del Fuego. Additionally, a smaller ozone hole has appeared over the Arctic. The thickness of the ozone layer is also decreasing in low latitudes, particularly in major industrial cities located in these areas (such as Moscow). The current trend shows a continued decrease in ozone concentration, with NASA reporting an average decline of 3% between 1969 and 1986.
Vertical division of the atmosphere
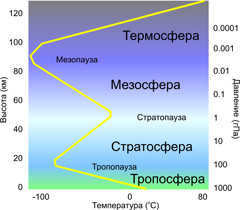
The atmosphere is divided into several layers (spheres) based on the vertical variations in meteorological parameters. One notable example is the vertical temperature changes, which occur hundreds of times faster compared to horizontal changes. These layers are determined by the variations in temperature, pressure, composition, electrical properties, and other characteristics of the air, as illustrated in Figure 1.1.
Fig. 1.1. The composition of the atmosphere: a – distribution of temperature (aT – in the tropics, ah.p. – in the cold polar zone); bT and bx – distribution of ozone in the tropics and polar zones; c – tropopause in the tropics; d – tropopause in the polar zone; e – subtropical jet stream; f – polar jet stream; g – stratopause; h – mesopause; i – turbopause; j – level of dissipation – release of H and H atomse; k – layer of stratospheric aerosols; l1, l2, l3, l4, l5 – tropospheric clouds: perispheric, high cumulus, cumulonimbus, cumulonimbus-rain, frontal clouds; e – exosphere
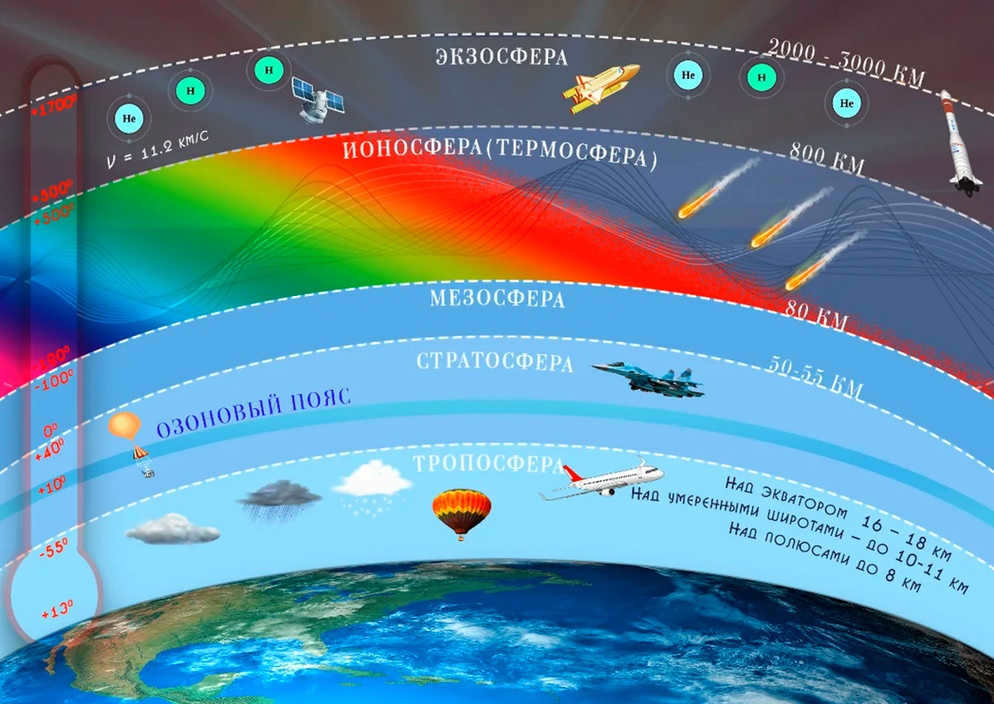
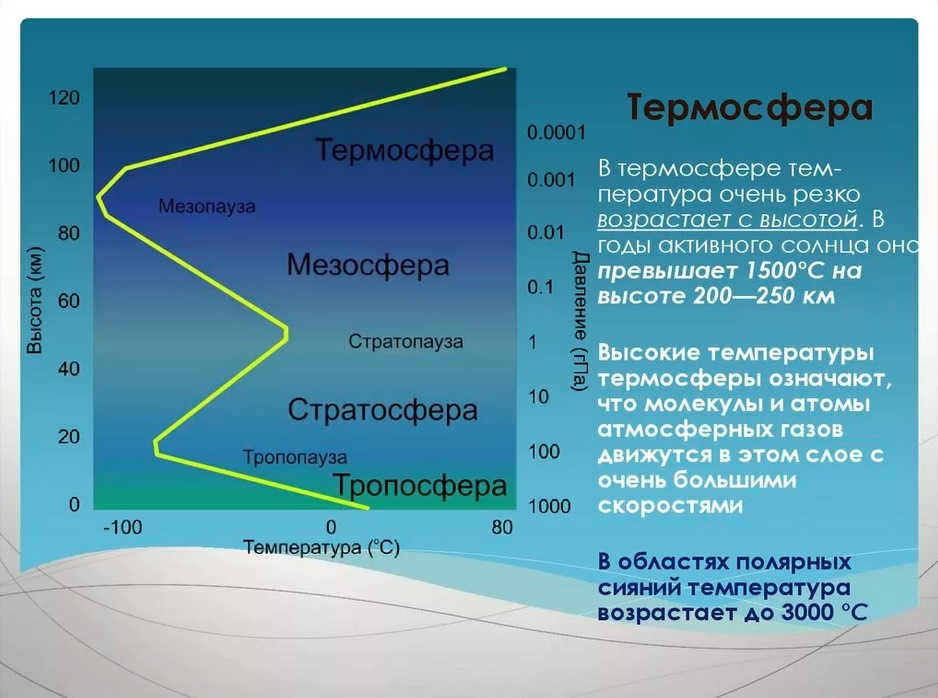
The air temperature distribution with altitude shows the most distinct difference between atmospheric layers. This characteristic allows us to identify five main spheres: the troposphere (which extends up to an average height of 11 km), the stratosphere (which spans from 11 to 50-55 km), the mesosphere (covering the range from 50-55 to 90 km), the thermosphere (reaching from 90 to 450 km), and the exosphere (which is located above 450 km). Additionally, there are interlayers with relatively small vertical extent between these layers, known as pauses. For instance, the transition from the troposphere to the stratosphere is marked by the presence of a tropopause. Similarly, the stratopause, mesopause, and thermopause are found between the other spheres, respectively.
The troposphere is the lowest layer of the Earth’s atmosphere, extending from the surface up to a certain height depending on various factors such as the time of year, latitude, and air circulation patterns. In temperate latitudes, the vertical extent of the troposphere is typically around 9-12 km, while it decreases to 8-10 km towards the poles and increases to 16-18 km towards the equator. The upper boundary of the troposphere descends over areas of high pressure and rises over areas of low pressure.
One of the defining characteristics of the troposphere is the presence of horizontal and vertical movements of air, as well as the intense mixing of air masses. This layer is also responsible for the majority of water vapor in the atmosphere, leading to cloud formation, precipitation, and other meteorological phenomena.
Another important feature of the troposphere is the decrease in temperature with increasing altitude. On average, the temperature decreases by around 0.65 °C for every 100 m of altitude. The mean annual temperature at the upper boundary of the troposphere varies depending on the location, with values of approximately -55 °C in temperate latitudes, -75 °C above the equator, -65 °C above the North Pole in winter, and -47 °C in summer.
The tropopause, which marks the transition between the troposphere and the stratosphere, can vary in vertical extent from a few hundred meters to 1-2 km. Close to the tropopause, narrow currents of air known as jet streams can be observed, with velocities ranging from 150-300 km/h. These jet streams play a significant role in atmospheric circulation patterns.
The stratosphere contains only small amounts of water vapor, which is why normal clouds do not form in this layer. However, occasionally, nacreous clouds can be observed at altitudes of 20-25 km. Additionally, the stratosphere experiences intense air circulation and vertical air movement.
Located above the stratopause is the mesosphere. From the surface up to an altitude of about 80 km, the temperature in the mesosphere decreases with altitude, reaching values as low as 90 °C. Observations from meteor probes and rockets indicate that wind speeds in the mesosphere can reach up to 150 m/s. The temperature decrease suggests the presence of strong air mixing in this layer. Silver clouds are sometimes observed in the mesosphere at heights of 82-85 km. Above the mesosphere lies the mesopause.
The thermosphere is located above it, where the temperature rises as you go higher. Indirect data and rocket observations indicate that at 150 km altitude, the temperature is about 220-240 K, at 200 km it reaches 500 K, and at the upper boundary of the thermosphere it exceeds 1000 K. The increase in temperature with altitude is attributed to the absorption of ultraviolet radiation by atomic oxygen and nitrogen. However, at these heights, the temperature only reflects the energy of molecular motion. An external object placed at this height does not experience such high temperatures due to the extremely thin air. The temperature of artificial satellites, spacecraft, and rockets at these heights is primarily influenced by the radiant energy they absorb.
The outer layer of the atmosphere, known as the exosphere or scattering sphere, extends from Earth’s corona to interplanetary space. As altitude increases, the temperature in the exosphere also rises, potentially reaching up to 2000 K. Due to the sparse nature of gases in the exosphere, their particles move at high speeds and rarely collide with each other.
Within the atmosphere, there exists a highly conductive layer that forms as a result of intense ionization caused by cosmic rays, ultraviolet radiation, and particle radiation from the Sun. This layer, known as the ionosphere, has a lower boundary at an altitude of 60-80 km and an upper boundary extending for several hundred kilometers.
Another important layer of the atmosphere, known as the ozonosphere, is located between 20 and 55 kilometers in altitude and contains the majority of ozone.
The atmosphere is divided into three parts based on its interaction with the Earth’s surface: the boundary layer, the lower layer, and the free atmosphere. The boundary layer experiences daily fluctuations in meteorological values. The surface layer, which is the lowest part of the boundary layer (50-100 m high), remains relatively stable in terms of heat and water vapor fluxes.
Horizontal Variability of the Troposphere
The troposphere exhibits heterogeneity not only in its vertical structure but also in its horizontal distribution. The distribution of meteorological variables is not uniform across the horizontal plane. The entire troposphere can be divided into large regions with relatively consistent weather conditions and narrower bands where there are abrupt changes in meteorological values.
Within the troposphere, there are large air masses that have relatively uniform properties and move within the general circulation of the atmosphere. These air masses are referred to as air masses.
In the Northern Hemisphere, the following air masses are identified:
1) Arctic air, which forms above the Arctic Circle, in the Arctic Basin, and over the surrounding continental areas;
3) Air of tropical origin is created in tropical and subtropical regions, and sometimes in southern temperate latitudes over the continent during the summer;
4) Equatorial air, which is formed in the equatorial zone and occasionally crosses from one hemisphere to another.
Depending on the surface where the air masses originate, they can be classified as either maritime or continental.
Air masses in the troposphere are constantly in motion, meaning they travel from the center of their formation to other regions.
Based on the classification related to temperature, air masses are categorized into cold and warm. A cold air mass is characterized by its ability to cool down the surrounding area upon its arrival. Typically, a cold mass moves towards a warmer surface beneath it. On the other hand, a warm air mass brings about warmth in the area it reaches. A warm mass, in contrast, moves towards a colder surface beneath it. The interaction between air masses occurs continuously.
Within the same air mass, meteorological values experience minimal changes. However, during the transition from one air mass to another, meteorological values undergo significant changes. The regions where neighboring air masses meet, resulting in rapid changes in meteorological values in the horizontal direction, are known as frontal zones or frontal surfaces. They are commonly referred to as fronts. The frontal zone is always inclined towards cold air, although the angle of inclination is only a few angular minutes.
Cold fronts and warm fronts are two types of fronts that occur in weather systems. A cold front occurs when a mass of cold air moves into an area and pushes under a mass of warm air that is retreating. This movement causes the warm air to be forced upward, creating a cold front. On the other hand, a warm front occurs when a mass of warm air moves over and gradually flows over a retreating mass of cold air. This movement creates a warm front.
In terms of air masses, there are three main fronts that can be distinguished based on geographical classification. These include the Arctic front, which occurs between Arctic and temperate air masses. The temperate front occurs between temperate and tropical air masses. Lastly, the tropical front occurs between tropical and equatorial air masses.
The layer of gas surrounding the Earth is known as the atmosphere, and the mixture of gases that compose it is referred to as air. The atmosphere is categorized into distinct layers based on its unique physical and chemical characteristics. So, what exactly are these layers that make up the Earth’s atmosphere?
Division of Atmospheric Layers by Gas Composition
The atmosphere is divided into two main layers based on gas composition: the homosphere and the heterosphere. The homosphere, which is the lower layer, has a uniform gas composition throughout. The upper boundary of the homosphere is at a height of 100 kilometers.
The heterosphere, on the other hand, extends from the top of the homosphere to the outer boundary of the atmosphere. In this layer, the gas composition is not uniform due to the effects of solar and cosmic radiation. These radiation sources cause the molecules in the heterosphere to break down into atoms through a process called photodissociation.
When molecules decompose into atoms in the heterosphere, they release charged particles – electrons and ions – which form a layer of ionized plasma known as the ionosphere. The ionosphere extends from the upper boundary of the homosphere to heights of 400-500 kilometers and possesses the ability to reflect radio waves, enabling radio communication.
At altitudes above 800 kilometers, the atmosphere’s light gas molecules start to escape into space, forming what is known as the exosphere.
Layers of the Atmosphere and Ozone Levels
The atmosphere contains the highest concentration of ozone (O3) at an altitude of 20-25 kilometers. This is a result of the abundant oxygen in the air and the presence of intense solar radiation. These specific layers of the atmosphere are referred to as the ozonosphere. Below the ozonosphere, the ozone levels in the atmosphere gradually decrease.
The prefix “exo” means “outside” and is also used to describe insects that have a tough outer shell or “exoskeleton,” like grasshoppers. The upper boundary of the exosphere marks the point where Earth’s gravity begins to weaken and interplanetary space commences.
The exosphere is the outermost layer of our atmosphere, and it separates the rest of the atmosphere from space. It has a thickness of approximately 6,213.7 miles (10,000 kilometers), which is nearly equivalent to the size of the Earth. Within the exosphere, there are various gases such as hydrogen and helium, although they are dispersed throughout.
Once you have learned fascinating information about the upper and lower boundaries of the exosphere, be sure to explore more than 20 astonishing facts about the mesosphere for children, as well as 40 remarkable and accurate facts about the troposphere.
The exosphere, the outermost layer of Earth’s atmosphere, is slowly vanishing into space. The atmosphere within the exosphere is incredibly tenuous and shares many similarities with a vacuum. Keep reading to discover some fascinating information about the exosphere:
The thermosphere, located just beneath the exosphere, is separated from it by the thermopause. The exobase is another term that can be used to refer to the lower surface of the exosphere. The lower boundary of the exosphere varies in altitude.
During the peak of the sunspot cycle, the Sun’s X-rays and ultraviolet radiation cause the thermosphere to expand, pushing the thermopause to a height of approximately 621.4 miles (1,000 kilometers) above sea level. This process is known as “inflation.” Conversely, during the low point of the sunspot cycle, when the Sun is less active, the radiation is less intense, and the thermopause recedes to a distance of 310.7 miles (500 kilometers) from Earth’s surface.
There is a debate among scientists regarding whether the exosphere should be considered part of the Earth’s atmosphere. Some scientists argue that the thermosphere constitutes the uppermost layer of the atmosphere, with the exosphere being entirely outside of it, in the realm of space. However, others argue that the exosphere should be regarded as an integral component of our planet’s atmosphere.
What is the number of layers in the Earth’s atmosphere?
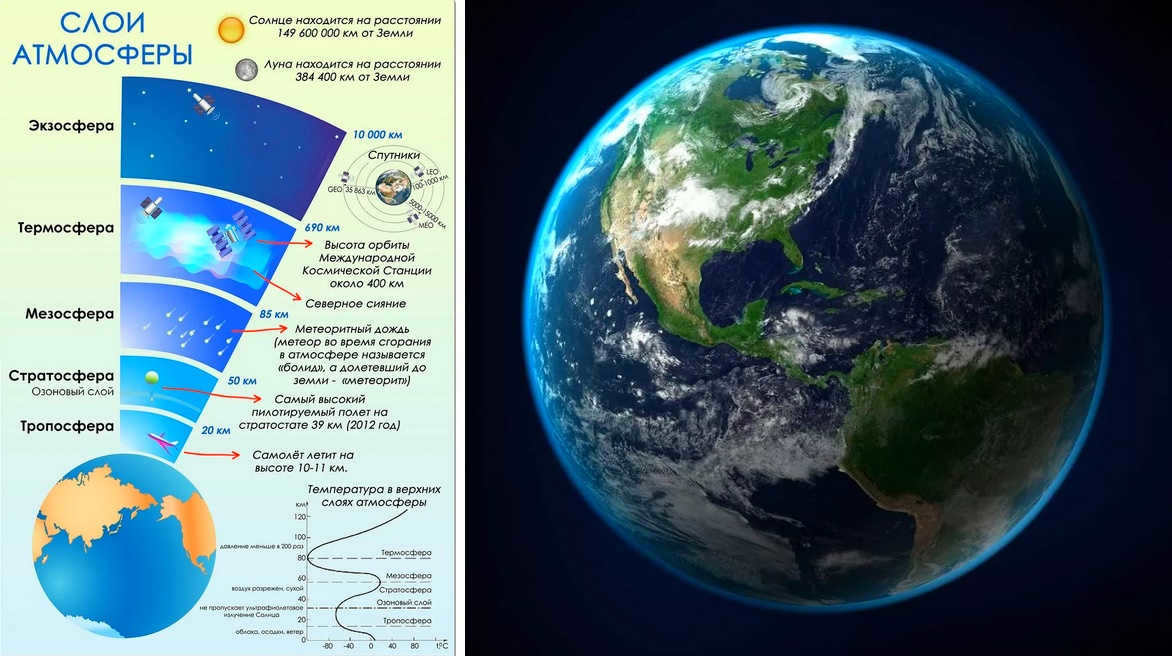
The Earth’s atmosphere is composed of five primary layers and numerous secondary layers. The main layers include the troposphere, stratosphere, mesosphere, thermosphere, and exosphere, arranged from lowest to highest in altitude.
Troposphere: The troposphere encompasses the region from the Earth’s surface up to approximately 7.5 miles (12 km) in altitude, with the altitude being lower at the poles and higher at the equator. Despite its relatively shallow depth, this layer plays a crucial role in sustaining life on Earth as it contains the necessary oxygen for both plants and animals to undergo photosynthesis. Additionally, the troposphere holds 99% of all water vapor and aerosols, which are minuscule solid or liquid particles suspended within the atmosphere. Due to the majority of heat being transferred from the Earth’s surface, the temperature within the troposphere generally decreases with increasing altitude. Moreover, it is worth noting that the troposphere represents the most densely populated layer of the atmosphere and experiences compression from the atmospheric pressure exerted above it.
Mesosphere: The mesosphere is located between 31.1 and 49.7 miles (50-80 km) above the Earth’s surface and experiences a decrease in temperature as altitude increases. Unique circumstances and specific times of day can result in the presence of extremely rare water vapor at the highest point of the mesosphere. This vapor is responsible for the formation of shimmering clouds, which are the highest clouds in the Earth’s atmosphere that can be observed without the aid of binoculars or a telescope. Additionally, the majority of meteors disintegrate while traversing through this atmospheric layer.
Thermosphere: Situated at an altitude of 80 to 700 kilometers above the Earth’s surface, the thermosphere encompasses the lowest portion of the ionosphere. The temperature in this layer increases in proportion to the relative density of molecules.
The uppermost layer of Earth’s atmosphere is known as the exosphere, reaching heights of 700 to 10,000 km above the planet’s surface. At its highest point, the exosphere merges with solar radiation. Due to the extremely low density of molecules in this layer, they do not exhibit typical gas behavior and instead escape into space.


Fascinating Information about the Exosphere
Discover some intriguing details about the exosphere that will enhance your understanding of the air in this outermost layer:
The exosphere is situated between 700 and 10,000 kilometers above the Earth’s surface.
Despite being the highest layer of the atmosphere, the exosphere serves as the planet’s first defense against solar radiation and cosmic rays. It is also the primary layer that interacts with meteorites, asteroids, and radiation, providing protection to our world.
The temperature in the exosphere varies greatly, ranging from 32°F (0°C) to over 3092°F (1700°C). It is cooler during the night and significantly hotter near the equator.
The exosphere is characterized by an extremely low density of air, primarily composed of helium and hydrogen. Trace amounts of other gases, such as atomic oxygen and carbon dioxide, can also be detected in this outermost region of the Earth’s atmosphere.
Gravity still exerts its influence on the exosphere, making it the farthest point from Earth where this force is still present. However, it should be noted that this distance is only halfway to the Moon and is considered accurate only in a technical sense.
If we define the boundary of the exosphere as the point where Earth’s gravity still affects it, then the exosphere would be considered the uppermost part of the planet’s atmosphere. However, many people consider the thermosphere, located approximately 6,213.7 miles (10,000 km) from the Earth’s surface, to be the most important part of the Earth’s atmosphere.
The geocorona refers to the portion of the exosphere that is visible from Earth.
On the outside of the world, the exosphere stretches into the black/blue zone, while the mesosphere stretches into the blue zone and the nebular stratosphere and troposphere closer to the bottom.
Due to the thinness of the air in the exosphere, molecules do not collide like they do in the lower atmosphere.
Here at Kidadl, we have meticulously prepared numerous fascinating family facts that will captivate everyone! If you found our suggestions for facts about the exosphere enjoyable, why not check out 20+ astonishing mesosphere facts for kids or 40 astonishing yet true facts about the troposphere?

Outer space is the region that lies beyond the Earth and its atmosphere, extending between celestial bodies. The temperature in outer space is primarily determined by the background radiation that originated from the Big Bang. This temperature is measured to be approximately 2.7 Kelvin, which is equivalent to about -271 °C in Celsius.

Composition of Earth’s Atmosphere
The layer of air surrounding our planet is known as the atmosphere. While other planets in our solar system also have atmospheres, none are as unique as Earth’s, which is capable of supporting life.
Gravity holds the atmosphere in place, and it is comprised of a variety of gases and other substances.
Originally, Earth’s atmosphere was vastly different from its current state. It primarily consisted of ammonia, methane, carbon dioxide, and water vapor, with very little, if any, free oxygen.
The concentration of gases in the atmosphere, and consequently the pressure, decreases as you ascend from the Earth’s surface.

The outermost layer of Earth’s atmosphere, located above a few hundred kilometers, is characterized by a remarkably sparse concentration of particles.
The Earth’s atmosphere can be divided into different layers, each with its own unique characteristics and properties.
- One of these layers is the troposphere, which is located closest to the Earth’s surface. The troposphere is about 12 kilometers thick, although this can vary slightly. In the troposphere, the temperature generally decreases as altitude increases. However, in some cases, there can be a temperature inversion, where the temperature actually increases with altitude. The troposphere is where weather formation primarily occurs, and it is marked by a boundary known as the tropopause.

- The layer above the tropopause is known as the stratosphere. Within this layer, the air becomes stratified, with denser and colder air positioned beneath warmer and lighter air. This stratification contributes to an increase in temperature as altitude increases. Due to its lack of turbulence, the stratosphere serves as the preferred altitude for most aircraft. As altitude increases, the air temperature continues to rise until it reaches approximately 10°C at an altitude of 48 km.
The primary factor behind the temperature increase at higher altitudes is the presence of a significant amount of ozone within the stratosphere. The interaction between ultraviolet light and ozone leads to a rise in temperature. The boundary between the stratosphere and the subsequent layer is referred to as the stratopause.

- After the stratopause, there is a subsequent decrease in temperature as altitude increases. This particular layer is known as the mesosphere. Near the mesopause at the highest point of the mesosphere, the temperature plummets to -90°C.

- The layer above the mesopause is known as the thermosphere, or the “warm layer”. In this region, the temperature rises as altitude increases, but the density of molecules is extremely low, even if we assume they are highly mobile.
- The exosphere, also known as the “outer layer,” is located above the thermosphere. The distinction between the two is not clearly defined. Within the exosphere, molecules possess sufficient kinetic energy to overcome the Earth’s gravitational pull and consequently venture into outer space.

- The ionosphere, also known as the outer region of the mesosphere and thermosphere, is characterized by the ionization of molecules and atoms caused by ultraviolet light and other high-energy particles. This altitude is where radio signals are reflected off of.
Temperature in the vacuum of space
What is the temperature like in the vacuum of space? It can reach extremes of approximately +255°C and -255°C when not exposed to direct sunlight. The concept of temperature is defined by a precise mathematical equation. It is directly proportional to the average kinetic energy of molecules in a substance. Kinetic energy is the energy that a substance possesses due to its motion. Even solid substances have molecules that exhibit random motion, resulting in kinetic energy and an associated temperature.

During the early stages of space exploration, determining the temperature in space was crucial for successful flights. It was essential to know the exact measurement in degrees Celsius to ensure the safety and functionality of the spacecraft.

Astronauts who venture into space experience extreme temperatures, resulting in exposure to solar radiation.
To ensure their safety, astronauts require spacesuits that can withstand temperatures ranging from -255 to +255°C, depending on the intensity of solar energy.
It is important to note that completely empty space lacks a specific temperature since there are no molecules present. In order for a substance to have a temperature rating, it must contain some form of matter. However, even in the vastness of interstellar space, there are still hydrogen molecules, electrons, and neutrons present, proving that space is not truly empty.

Despite the absence of particles in that region, the expanse is permeated by cosmic microwave background radiation (CMB). This “radiation” saturates the entirety of the cosmos and is believed to be the residual energy from the initial explosion of the Big Bang. The universe was immensely scorching during the inception of the Big Bang.

Originally, there existed an immense quantity of radiation composed of particles known as photons, which are commonly referred to as “particles of electromagnetic radiation”. Nevertheless, due to the expansion of the Universe, space began to cool down. At present, the radiation has significantly decreased in temperature.

MFI is composed of photons with varying energies. The MFI temperature is equivalent to 2.7 degrees Kelvin, which is approximately -270°C and just 2.7 degrees above absolute zero.

Hence, the temperature of 2.7 K in the MFI can be referred to as the “space temperature” – a representation of the energy present in vacuum. Naturally, 2.7 K is quite a chilly value!
Protection against temperature fluctuations in the vacuum of space
The Earth’s atmosphere effectively regulates the distribution of solar heat by means of conduction, convection, and radiation. This is why we feel temperature changes so acutely on our planet.
Due to sunlight or weather conditions, particles in space move slightly faster because the temperature in orbit is -273.15 degrees Celsius.

What is the temperature outside the International Space Station orbiting Earth?
The temperature near the ISS on the day side is approximately +4°C. However, in the shadow of the Earth, the temperature plummets to around -160°C.
Consequently, astronauts venturing beyond the secure confines of our planet don insulating spacesuits that provide protection against extreme temperatures.

As an illustration, the heating systems of spacesuits during the Apollo era featured flexible coils and lithium batteries. In contrast, contemporary spacesuits are furnished with minuscule chemical beads that respond to varying temperatures, thereby safeguarding astronauts against both extreme cold and hot conditions.

The portable life support system integrated into the Artemis spacesuits is designed to assist astronauts in maintaining optimal temperatures during lunar expeditions in 2024 and beyond.

Coldest Temperature in Outer Space
The typical temperature in the vicinity of Earth’s outer space is 10.17 degrees Celsius.
In the vast expanse of interstellar space, the temperature plummets to a mere 3 degrees Kelvin, just slightly higher than absolute zero, the frigid benchmark for coldness.

The Boomerang Nebula, situated in the Centaurus constellation, holds the title for being the coldest known object in the entire universe. This remarkable celestial body is located approximately 5,000 light-years away from our home planet, Earth.





