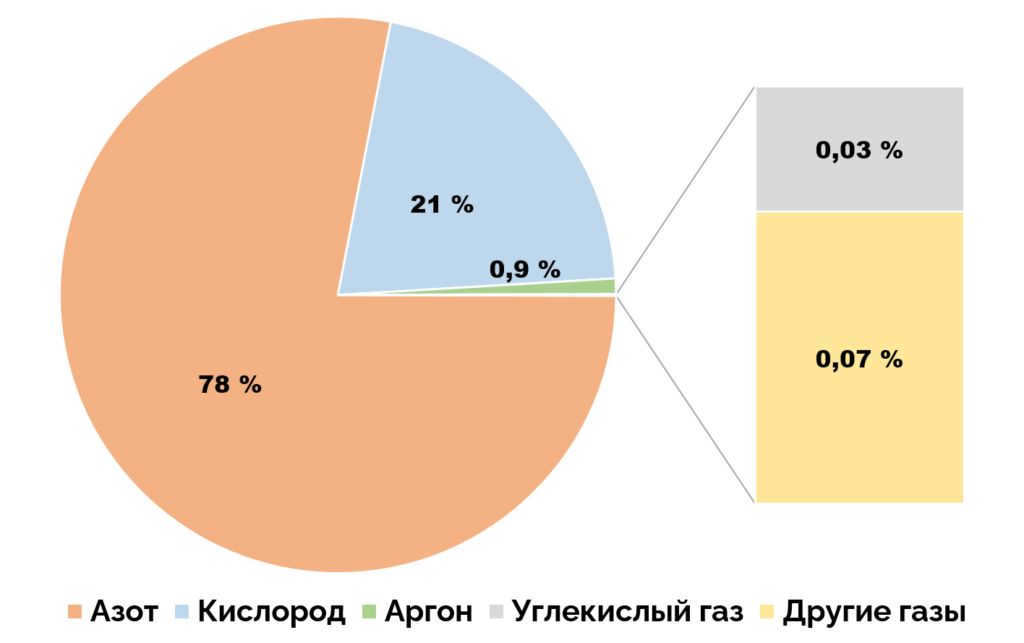
The gaseous shell surrounding the Earth, known as the atmosphere, is made up of air. Air is composed of various gases, with nitrogen making up 78.087%, oxygen making up 20.95%, argon making up 0.93%, carbon dioxide making up 0.041%, and small amounts of other gases present. The atmosphere plays a crucial role in protecting life on Earth by filtering out harmful ultraviolet solar radiation, regulating surface temperature through the greenhouse effect, and moderating the temperature difference between day and night.
These cloudy formations, which can alternate between liquid and solid states, are not typically classified as part of the atmosphere. However, the water vapor present in humid air makes up an average of 0.25% of the atmosphere’s total mass. Vapor possesses a notable characteristic in that it is the only liquid within the atmosphere that can rapidly transition between phases (solid, liquid, gas), primarily based on temperature and concentration, both of which vary greatly across time and space. As heat increases, the air and its moisture ascend, causing atmospheric pressure and temperature to decrease at higher altitudes.
Summary
- 1 Description
- 2 Detailed chemical composition
- 2.1 Main constituents
- 2.2 Changes in CO levels 2
- 3.1 Troposphere
- 3.2 Ozone layer
- 3.3 Stratosphere
- 3.4 Mesosphere
- 3.5 Thermosphere
- 3.6 Ionosphere
- 3.7 Exosphere
- 6.1 Wave Propagation
- 6.2 Optical absorption
- 6.3 Emission
- 11.1 Notes
- 11.2 References
- 12.1 Bibliography
- 12.2 Filmography
- 12.3 Related articles
- 12.4 External links
Description
The boundary between the Earth’s atmosphere and space is not clearly defined, as it gradually thins out and fades into space. However, by observing changes in the density of gases surrounding the Earth, it can be determined that the average thickness of the atmosphere, denoted as d’, ranges from 350 to 800 km, depending on solar activity. This range corresponds to the transition between the thermosphere and the exosphere.
Various parameters have been used to define the limit of the Earth’s atmosphere in the literature. For example, some define it as 31 km, which is the altitude below which 99% of the atmosphere’s mass is concentrated. Others define it as 80 km, which marks the base of the ionosphere. Another definition places the limit at 1,000 km, beyond which the density of gases is no longer significantly influenced by solar winds. Finally, some consider the exosphere to be the outermost layer of the atmosphere, with its limit estimated at 50,000 km.
The Fédération Aéronautique Internationale considers the Karman line, located at 100 km, as the demarcation between the Earth’s atmosphere and outer space.
At an altitude of 120 km, observable atmospheric effects start to become noticeable during re-entry.
The precise definition of the boundary between Earth’s atmosphere and the solar atmosphere is not clear-cut: the outer limit of the atmosphere is determined by the point at which atmospheric gas molecules no longer experience the gravitational pull of Earth and the interactions of its magnetic field. This occurs at different altitudes depending on the latitude, approximately 60 km above the equator and 30 km above the poles. However, these altitude values are only approximate as the Earth’s magnetic field is constantly distorted by the solar wind. As a result, the thickness of the atmosphere can vary significantly. Additionally, similar to the oceans, the atmosphere is influenced by the rotation of the Earth-Moon system and the gravitational interaction between the Moon and the Sun. Due to the lighter and less tightly bound nature of gas molecules compared to seawater molecules, atmospheric tides are a much more prominent phenomenon than oceanic tides.
The majority of the atmospheric mass is located near the surface: as you go higher in altitude, the amount of air decreases and the pressure decreases as well; this can be quantified using an altimeter or barometer.
The atmosphere plays a crucial role in the greenhouse effect, which helps to warm the Earth’s surface. Without the atmosphere, the average temperature on Earth would be -18°C, in comparison to the current temperature of 15°C. The greenhouse effect occurs due to the specific properties of gases in relation to electromagnetic waves.
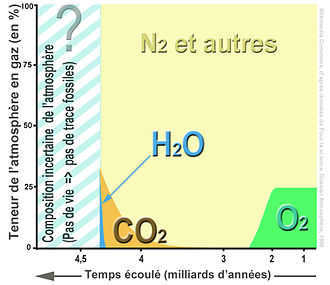
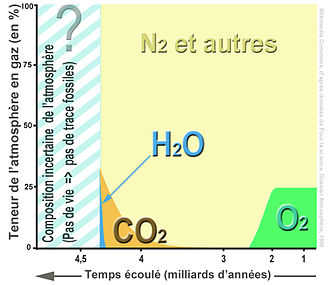
There have been significant fluctuations in the known levels of CO2 in the Earth’s atmosphere. In order to highlight these changes, a non-linear time scale is used.
Over time, the levels of CO2 have undergone dramatic transformations.
The gases in the atmosphere are in constant motion, leading to a heterogeneous composition and physical characteristics. The concentrations of minor components, including pollutants, vary greatly across the globe due to localized sources of emissions. These sources can be either human activities (such as factories and indoor/outdoor air pollution) or natural processes (like geothermal energy and organic matter decomposition).
Main constituents
At sea level, the composition of dry air primarily consists of 78.1% nitrogen and 20.9% oxygen. The remaining 1% is predominantly comprised of 0.93% argon and 0.04% carbon dioxide. It also contains trace amounts of other chemical elements, minor gases whose proportion varies with altitude. These components constitute less than 0.03% of the atmosphere and mainly consist of inert gases such as neon, helium, krypton, xenon, and radon. Among these constituents, the greenhouse gases include water vapor, carbon dioxide, methane, nitrogen oxide, and ozone.
ppmm: parts per million by mass
| Nitric oxide (NO) | 0.5 parts per million by volume |
| Nitrous oxide (N 2 O) | 0.3 parts per million by volume |
| Xenon (Xe) | 0.09 parts per million by volume |
| Ozone (O 3 ) | ≤ 0.07 parts per million by volume |
| Nitrogen dioxide (NO 2 ) | 0.02 parts per million by volume |
| Iodine (I 2 ) | 0.01 parts per million by volume |
| Carbon monoxide (CO) | 0.2 parts per million by volume |
| Ammonia (NH 3 ) | traces |
Smaller quantities of other elements that occur naturally are also present, including dust (like Saharan airborne dust), pollen and spores, as well as viruses and bacteria. The air also contains a high amount of aerosols that originate from either natural or human activities, along with pollutants. These pollutants include CO (contrary to popular belief, CO2 is not an air pollutant, but rather a greenhouse gas with negligible direct health effects), particulate matter, nitrogen oxides, chlorine (either in its elemental form or in compounds), fluorine (in compounds), mercury, and sulfur (in compounds such as SO2). Agricultural areas also contribute to the presence of methane (through slurry fermentation and rice fields), pesticides (which may vary in their solubility in air or air humidity, depending on their vapor pressure), and nitrogen (from fertilizers). Additionally, rockets and airplanes contribute to air pollution by burning their fuel.
Evolution of Carbon Dioxide Levels
Between 800,000 years ago and the beginning of the industrial revolution, the concentration of carbon dioxide (CO2) in the atmosphere fluctuated between 180 and 280 parts per million (ppm). These minimum and maximum values corresponded to the glacial and interglacial periods, respectively. Currently, the concentration of CO2 is increasing at a rate a hundred times faster than it did at the end of the last ice age 10,000 years ago. It is now reaching levels that vary from year to year, with an average increase of about +2.1 ppm/year compared to an average increase of +16 parts per billion (ppb) (0.016 ppm/year) during the same time period.
By the end of the 1950s, there was a rise of +0.7 ppm per year, which was one-third of the present rate. It is undeniable that concentrations higher than the current level were present approximately fifty million years ago; nevertheless, based on available data, the yearly fluctuation has never reached the current level in recent times.
The mole fraction of carbon dioxide experienced an increase in January 2017, rising from 0 to 0.040% or 404 ppm, whereas in 1998, it was only 345 ppm.
- Pliocene (-5 to -3 million years ago): 415 parts per million, ranging from 350 to 450 parts per million (with an average of 400 parts per million)
- Stage 11 (-400,000 years ago): 290 parts per million
- Before the industrial revolution (prior to the 19th century): 280 ppm
- 1958: 315 ppm
- 1974: 330 ppm
- 2001: 370 ppm
- June 2005: 382 ppm
- 2013: 395 parts per million
- 2015: 400 parts per million
- 2020: 415 ppm
Composition
Composition is the arrangement of elements in a work of art. It refers to how the different parts of a piece are organized and relate to one another. A well-composed artwork is visually appealing and effectively communicates the artist’s intended message or idea.
There are many different principles and techniques that artists use to create a strong composition. These include the rule of thirds, balance, symmetry, and contrast. Each of these principles helps to create a sense of harmony and unity in the artwork.
One important aspect of composition is the focal point. This is the area of the artwork that draws the viewer’s attention and is usually the most important part of the piece. The focal point can be created through the use of color, contrast, or placement within the composition.
Another important aspect of composition is the use of negative space. Negative space refers to the empty or unoccupied areas of a composition. It can be used to create a sense of balance and help draw attention to the focal point.
Overall, composition plays a crucial role in the success of an artwork. It is important for artists to carefully consider how they arrange the elements within their pieces to create a visually pleasing and effective composition.
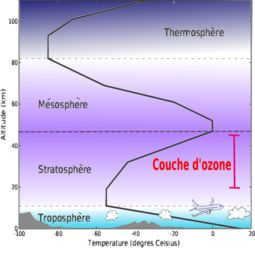
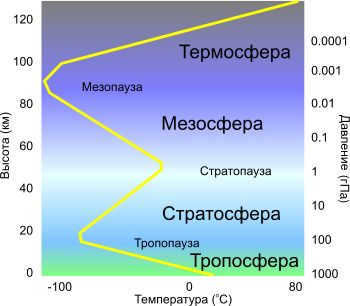
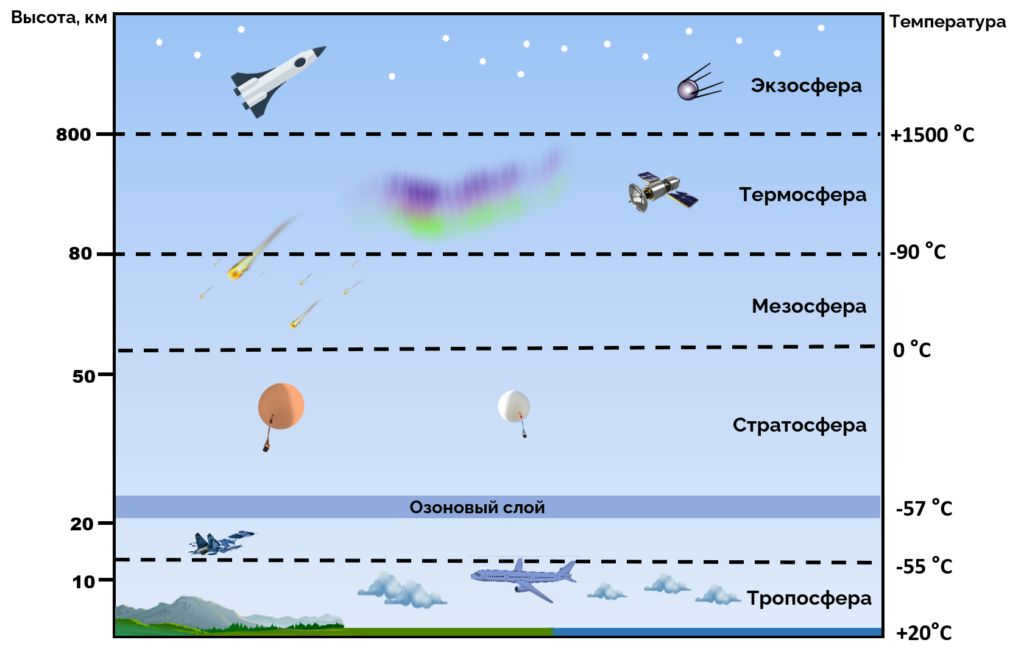
The atmosphere is separated into numerous layers of different significance, and their boundaries are determined by changes in temperature at different altitudes. Starting from the lowest layer and going upwards:
The temperature in the troposphere decreases as you go higher in altitude, ranging from the Earth’s surface to about 8 to 15 km. This layer is thicker near the equator, measuring about 13 to 16 km, and thinner near the poles, measuring about 7 to 8 km. The troposphere contains the majority of the Earth’s air mass, about 80 to 90%, and nearly all of the water vapor. It is where weather phenomena like clouds and rain occur, as well as horizontal and vertical movements of the atmosphere, such as thermal convection and winds. Moving up to the stratosphere, the temperature actually increases with altitude until it reaches 0°C at around 15 km to 50 km. The stratosphere is home to a significant portion of the ozone layer. Next is the mesosphere, where the temperature decreases with altitude from about 50 km to 80 km, reaching as low as -80°C. Finally, the thermosphere experiences an increase in temperature as you go higher from 80 km to about 350 to 800 km. Beyond that, the exosphere stretches from 350 km to 800 km above sea level, with the highest point reaching about 50,000 km above sea level.
The Layer of Atmosphere Called Troposphere
Please note that the lower portion of the troposphere is commonly referred to as the Peplos. This particular layer, which extends up to approximately 3 km, is also known as a “dirty” layer due to its high concentration of impurities (such as aerosol or nuclei) that serve as the nuclei for water droplet formation when the air reaches 100% relative humidity. The Peplos layer comes to an end with the presence of the ash layer. The existence of this contaminated layer helps explain why there is a lack of supersaturated air in the upper troposphere.
Moving on to the tropopause, this is the boundary that separates the troposphere from the stratosphere.
Ozone layer
The ozone layer, which is located in the Earth’s stratosphere, plays a crucial role in protecting life on our planet. It acts as a shield, absorbing the majority of the Sun’s ultraviolet (UV) radiation, preventing it from reaching the Earth’s surface. This layer is made up of ozone molecules, which are formed when oxygen molecules are broken apart by UV radiation and then recombine. However, human activities have led to the depletion of the ozone layer, particularly through the release of chlorofluorocarbons (CFCs) and other ozone-depleting substances. The use of CFCs in refrigeration, air conditioning, and aerosol propellants has contributed to the thinning of the ozone layer, creating what is commonly known as the “ozone hole.” This hole allows more UV radiation to reach the Earth’s surface, which can have harmful effects on human health and the environment. In response to this issue, the international community has taken steps to reduce the production and use of ozone-depleting substances. The Montreal Protocol, signed in 1987, has been successful in phasing out the use of CFCs and other harmful substances, leading to the gradual recovery of the ozone layer. However, continued efforts are needed to ensure the long-term protection of this vital layer.
While the stratosphere includes the ozone layer, the latter is considered a distinct layer due to its unique chemical and physical makeup. The ozone (O3) in Earth’s stratosphere is formed when oxygen molecules (O2) are split into separate atoms by ultraviolet light, which then combine with other oxygen molecules (O2) to create ozone (O3). Although O3 is unstable, it has a longer lifespan in the stratosphere. However, when it is exposed to ultraviolet light, it breaks down into O2 and O. This continuous process is known as the ozone-oxygen cycle and occurs in the ozone layer, which is located 10 to 50 km above the Earth’s surface. Nearly 90% of the ozone in the atmosphere is found in the stratosphere. The highest concentrations of ozone are typically found at altitudes between 20 and 40 km, ranging from 2 to 8 parts per million.
Stratosphere
The stratosphere is a layer of the Earth’s atmosphere that extends from the tropopause, which is located approximately 7 to 17 km above the Earth’s surface, all the way up to about 50 km in altitude. It is characterized by an increase in temperature with altitude and is home to the majority of the Earth’s ozone layer.
Stratopause, on the other hand, is the boundary that separates the stratosphere from the mesosphere. It can be found at an altitude of around 50 to 55 km above sea level. At this point, the atmospheric pressure is approximately 1/1000th of the pressure at sea level.
Mesosphere
The mesosphere, which is derived from the Greek word μέσος meaning “middle,” spans from 50 km to 80 to 85 km. As altitude increases, the temperature drops once again, reaching -100 °C (173.1 K) at the highest point of the mesosphere. This region is where the majority of meteoroids burn up upon entering the Earth’s atmosphere. Additionally, the mesosphere can alter the trajectory and characteristics (such as mass and orbit) of moving objects, as demonstrated during the incident on October 13, 1990.
Mesopause refers to the point of transition between the mesosphere and the thermosphere, and it is where the lowest temperature in the mesosphere is found. Remarkably, it is the coldest location on our planet, with temperatures reaching -100 °C (173.1 K).
Thermosphere
The thermosphere is a region of the atmosphere that begins at approximately 80 to 85 km above the Earth’s surface and extends up to an altitude of 640 km. This layer of the atmosphere is characterized by an increase in temperature with increasing altitude. Despite temperatures reaching as high as 1500 ° C, humans would not perceive this heat due to the extremely low air pressure. The International Space Station orbits within this layer, maintaining an altitude of around 350 to 400 km. The Space Studies Committee recommends using the MSIS-86 model as a general representation of the thermosphere.
Thermopause
The thermopause is the upper boundary of the thermosphere, with its height ranging from 500 to 1000 km.
The ionosphere, which is the region of the atmosphere that is ionized by solar radiation, spans from 60 to 800 km and is composed of three distinct layers:
- Layer D (60 to 90 km)
- Layer E (90 to 120 km)
- Layer F (120 to 800 km), encompassing the thermosphere and exosphere. This layer plays a significant role in atmospheric electricity and serves as the inner boundary of the magnetosphere. Its impact extends to practical applications, such as the modulation of radio wave propagation on Earth. Additionally, it is the site where phenomena like the aurora borealis and transient luminous events associated with thunderstorms occur.
The Uppermost Layer of Earth’s Atmosphere: Exosphere

The exosphere commences at the exobase, also referred to as the “critical level,” at approximately 500-1000 km and stretches beyond 10,000 km above the Earth’s surface. Within this region, there exist particles that freely circulate and either migrate from the magnetosphere or originate from the solar wind.
Pressure and thickness
At sea level, the average atmospheric pressure is 1,013.25 hectopascals, while the total mass of the atmosphere is approximately 5.148 × 10 18 kg.
If the density of the atmosphere were to remain constant as altitude increases, the atmosphere would suddenly collapse at approximately 7.81 km. However, the density actually decreases with altitude, already having decreased by 50% at 5.6 km. To put this into perspective, the highest mountain, Mount Everest, stands at an altitude of 8.8 km, meaning that at its peak the air density is less than 50% of what it is at sea level.
This decrease in pressure follows an almost exponential pattern, with the pressure halving every 5.6 km and decreasing by 63.2% every 7.64 km (which is the average height of Earth’s atmosphere, below 70 km). Even in the exosphere, the atmosphere is still present, as evidenced by the drag experienced by satellites. (1 – 1/e is equal to 1 – 0.368, which equals 0.632)
The pressure equations at different altitudes can be used to estimate the thickness of the atmosphere. Here is some reference data:
- Approximately 50% of the total mass of the atmosphere is located below an altitude of 5.6 km above sea level;
- About 90% of the total mass of the atmosphere is located below an altitude of 16 km. Currently, commercial air transportation operates at an altitude of around 10 km, while the summit of Mount Everest reaches an altitude of 8,849 m. In the upper regions of the atmosphere, where there are fewer gases, phenomena such as polar lights, dawn, and other atmospheric effects can be observed. The highest altitude ever reached by an X-15 airplane in 1963 was 108 km.
Mass and Density: A Closer Look
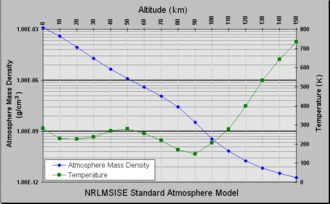
The standardized atmospheric model NRLMSISE-00 provides information on temperature and density variations relative to altitude.
At sea level, the density of air is approximately 1.2 kg/m3 (1.2 g/L). The density of air naturally changes with altitude and weather conditions. These variations are relatively minor in populated areas, but they become more significant in the upper atmosphere and space due to the effects of solar radiation.
As altitude increases, atmospheric density decreases. This phenomenon can be accurately described using the barometric leveling formula. Meteorologists and space agencies use more advanced models to forecast weather and track the gradual decay of satellite orbits.
Opacity
The concept of opacity refers to the amount of solar radiation (or sunlight) that is received by the Earth from the Sun. The Earth, in turn, emits radiation back into space, but at longer wavelengths that are invisible to the human eye. The atmosphere plays a crucial role in determining the opacity of this radiation, as it can either prevent radiation from entering the atmosphere or trap it within. Two significant factors that contribute to the opacity of the atmosphere are clouds and the greenhouse effect.
Propagation of Waves
When light passes through the atmosphere, it interacts with the atmosphere by scattering waves. If the light does not interact with the atmosphere, it is considered direct radiation, which is like looking directly at the sun. Indirect radiation refers to light that has been scattered in the atmosphere. For instance, on a cloudy day when shadows are not visible, there is no direct radiation to cast them because the light has been scattered. Another example is the phenomenon of Rayleigh scattering, where shorter wavelengths (blue) are more easily scattered than longer wavelengths (red). This is why the sky appears blue, as blue light is scattered. It is also why sunsets appear red. As the sun approaches the horizon, its rays pass through more of the atmosphere than usual before reaching our eyes. As a result, all the blue light is scattered, leaving only the red light in the setting sun.
Primary menu
Trending Articles
Air Layer
An air layer is the gaseous blanket (geosphere) encompassing our planet Earth. Its inner boundary encompasses the hydrosphere and a portion of the Earth’s crust, while its outer boundary marks the edge of the near-Earth region of outer space.
The entirety of the fields of physics and chemistry that investigate the air layer is known as atmospheric physics. The air layer influences the weather conditions on the Earth’s surface; meteorology is concerned with the examination of weather patterns, while climatology focuses on long-term fluctuations in climate.
Troposphere
The troposphere is the lowermost layer of the Earth’s atmosphere, extending from the surface up to an altitude of 8-10 km in polar latitudes, 10-12 km in temperate latitudes, and 16-18 km in tropical latitudes. Its upper boundary varies depending on the season, being lower in winter and higher in summer.
This layer contains over 80% of the total mass of atmospheric air and approximately 90% of all water vapor present in the atmosphere. It is the layer where turbulence and convection are most strongly developed, leading to the formation of clouds and the development of cyclones and anticyclones.
The temperature in the troposphere decreases with increasing altitude, with an average vertical gradient of 0.65°C per 100 meters of ascent.
At the Earth’s surface, certain conditions are commonly referred to as “normal conditions” for engineering purposes. These conditions include a density of 1.2 kg/m3, a barometric pressure of 101.34 kPa, a temperature of 20°C, and a relative humidity of 50%. These conventions are used as standard references in engineering calculations.
Tropopause
The layer of transition between the troposphere and the stratosphere. The tropopause has a variable thickness, ranging from a few hundred meters to 2-3 kilometers.
Stratosphere
The stratosphere begins at an altitude of 50-55 kilometers. As you go higher, the temperature rises to approximately 0 °C. The stratosphere experiences low levels of turbulence and has a minimal amount of water vapor. Additionally, it has a higher concentration of ozone compared to the layers below and above it, with the maximum concentration occurring at altitudes of 20-25 kilometers.
Stratopause
The stratopause is the layer of the atmosphere located between the stratosphere and the mesosphere. It is characterized by a maximum in the vertical temperature distribution, with temperatures around 0°C.
Mesosphere
The mesosphere is the layer of the atmosphere above the stratopause, extending to an altitude of 80-85 km. In this region, temperature decreases with altitude, with an average vertical gradient of (0.25-0.3)°C per 100 meters. The main energy process in the mesosphere is radiant heat exchange. Additionally, complex photochemical processes involving free radicals, vibrationally excited molecules, and other factors contribute to atmospheric luminescence.
Mesopause
The mesopause is a transitional layer that separates the mesosphere from the thermosphere. In terms of temperature, there is a minimum point at approximately -90°C in the vertical temperature distribution.
Thermosphere
The thermosphere extends up to an altitude of about 800 km. As you go higher, the temperature gradually increases, reaching values of around 1500 K at heights of 200-300 km. Beyond this point, the temperature remains relatively constant at high altitudes. The air in the thermosphere gets ionized due to the effects of ultraviolet and X-ray solar radiation, as well as cosmic radiation. This ionization leads to phenomena such as the “polar lights” or auroras, which are primarily observed in the ionosphere. At altitudes above 300 km, atomic oxygen becomes the dominant component of the atmosphere in this region.
Exosphere (scattering sphere)
The outer layer of the atmosphere, known as the exosphere or scattering sphere, is where fast-moving light hydrogen atoms have the ability to escape into outer space. This region of the atmosphere is characterized by temperatures that can reach levels of over 3000 K. At distances far from the Earth, typically around 2-3 thousand km or more, the exosphere is primarily composed of neutral hydrogen atoms. However, as we move to lower altitudes, helium atoms become more prevalent. Additionally, at even lower altitudes, oxygen atoms also contribute to the composition of the exosphere.
The atmosphere’s thickness extends approximately 2000 – 3000 km above the Earth’s surface. The overall mass of air amounts to (5.1-5.3)×1018 kg. The molecular weight of pure, dry air is 28.966. At sea level and 0 °C, the pressure measures 101.325 kPa. The critical temperature is -140.7 °C, while the critical pressure is 3.7 MPa. The specific heat capacity (Cp) is 1.0048×10³ J/(kg-K) at 0 °C, and the specific heat capacity at constant volume (Cv) ranges from 0.7159-10³ J/(kg-K) at 0 °C. Air’s solubility in water reaches 0.036% at 0 °C and 0.22% at 25 °C.
The Earth’s atmosphere primarily consists of gases and various impurities, such as dust, water droplets, ice crystals, sea salts, and combustion products.
The concentration of gases in the atmosphere remains relatively stable, except for water (H2O) and carbon dioxide (CO2).
History of the formation of the atmosphere
Currently, the scientific community does not have a complete understanding of all the precise stages that led to the formation of Earth’s atmosphere. The prevailing theory suggests that the composition of the atmosphere has gone through four distinct phases over time. Initially, the atmosphere was primarily made up of light gases such as hydrogen and helium that were captured from interplanetary space. This initial phase is referred to as the primary atmosphere and is estimated to have existed approximately four billion years ago. The next phase involved intense volcanic activity, which introduced gases such as carbon dioxide, ammonia, and water vapor, resulting in the formation of a secondary atmosphere. This secondary atmosphere, which existed approximately three billion years ago, was characterized by a high concentration of these additional gases. The formation of the atmosphere during this period was influenced by several factors, including the constant leakage of hydrogen into interplanetary space.
The atmosphere undergoes chemical reactions when exposed to ultraviolet radiation, thunderstorms, and other factors.
Over time, these factors have contributed to the development of the tertiary atmosphere, which is characterized by a lower hydrogen content and a higher nitrogen and carbon dioxide content (formed through chemical reactions involving ammonia and hydrocarbons).
The emergence of life and the presence of oxygen
With the appearance of living organisms on Earth, the composition of the atmosphere began to change as a result of photosynthesis, which releases oxygen and absorbs carbon dioxide. However, there is evidence (through analysis of the isotopic composition of atmospheric oxygen and oxygen released during photosynthesis) that supports the idea that atmospheric oxygen has a geological origin.
At first, the consumption of oxygen occurred for the purpose of oxidizing reduced compounds such as hydrocarbons and the oxide form of iron found in the oceans. As a result, the oxygen content in the atmosphere remained relatively stable during this period. However, towards the end of this stage, there was a noticeable increase in atmospheric oxygen levels. This gradual rise in oxygen ultimately led to the formation of the modern atmosphere with its oxidizing properties.
Throughout the Phanerozoic era, there were fluctuations in the composition and oxygen levels of the atmosphere. These changes were closely tied to the rate at which organic sedimentary rocks were deposited. Consequently, during periods of coal accumulation, it is believed that the oxygen content in the atmosphere surpassed present-day levels. Additionally, there may have been an increase in carbon dioxide levels during periods of intense volcanic activity. More recently, human activities have had a significant impact on the evolution of the atmosphere. The combustion of hydrocarbon fuels from previous geological epochs has led to a constant and significant increase in carbon dioxide levels in the atmosphere.
All of the processes that have shaped the Earth’s atmosphere continue to be active today. However, in the short term, there are other factors that also play a crucial role.
Contrary to common misconception, forests have little effect on the oxygen and nitrogen levels in the atmosphere. Oxygen is not accumulated by forests, but rather absorbed, while the taiga actually releases oxygen. The carbon dioxide levels in the atmosphere are primarily influenced by swamps and oceans. These areas collect organic matter, which over geological time is converted into coal, oil, and gas.
In the 1990s, experiments were conducted to create a closed ecological system known as “Biosphere 2.” However, this experiment failed to establish a stable system with a consistent air composition. The presence of microorganisms caused a decrease in oxygen levels and an increase in carbon dioxide.
The production of large quantities of N2 is caused by the oxidation of the primary ammonia-hydrogen atmosphere by molecular O2, which started to be released from the surface of the planet due to photosynthesis, believed to have occurred about 3 billion years ago (according to another theory, the oxygen in the atmosphere has a geological origin). Nitrogen is oxidized to NO in the upper atmosphere, utilized in industry, and fixed by nitrogen-fixing bacteria, while N2 is released into the atmosphere as a result of denitrification of nitrates and other nitrogen-containing compounds.
N2 nitrogen is an inert gas and only reacts under specific conditions (such as lightning discharge). It can be oxidized and transformed into a biological form by cyanobacteria and certain bacteria (such as nodule bacteria that form a symbiotic relationship with leguminous plants).
The origin of noble gases such as argon, helium, and krypton can be traced back to volcanic eruptions and the decay of radioactive elements. In comparison to space, the Earth and its atmosphere are generally lacking in noble gases. This is believed to be due to the continuous leakage of gases into interplanetary space.
Photosynthesis and the absorption of vast amounts of CO2 by the world’s oceans play a significant role in regulating the levels of this gas in the atmosphere. CO2 enters the atmosphere through various processes, including the decomposition of carbonate rocks and organic matter from plants and animals, as well as through volcanism and human activities. Over the past century, the concentration of CO2 in the atmosphere has increased by 10%, with the majority (360 billion tons) originating from the combustion of fossil fuels. If the rate of fuel combustion continues to grow, the amount of CO2 in the atmosphere is projected to double within the next 50-60 years, potentially leading to global climate change.
Fuel combustion is the primary source of harmful gases (CO, NO, SO2). The oxidation of sulfur dioxide by O2 in the atmosphere results in the formation of SO3 in the upper atmosphere. This compound then reacts with H2O and NH3 vapors, producing H2SO4 and (NH4)2SO4, which are subsequently deposited on Earth’s surface through precipitation. The use of internal combustion engines contributes to significant air pollution through the emission of nitrogen oxides, hydrocarbons, and lead compounds.
Aerosol pollution in the atmosphere can arise from both natural phenomena (such as volcanic eruptions, dust storms, the evaporation of sea water droplets, and the release of plant pollen) and human activities (such as mining, fuel combustion, and cement production). The extensive release of solid particles into the atmosphere may be one of the factors influencing climate change on our planet.
Characterizing and understanding the structure of the atmosphere is crucial for studying weather and climate. The atmosphere is composed of various elements such as air density, pressure, temperature, and composition, which all play a role in determining its physical state. As altitude increases, both air density and atmospheric pressure decrease. The atmosphere can be divided into different layers based on temperature and electrical properties, each with its own unique characteristics. These layers include the troposphere, stratosphere, mesosphere, thermosphere, and exosphere. The boundaries between these layers are marked by transitional regions known as the tropopause, stratopause, and so on.
The troposphere, which is the most extensively studied layer of the atmosphere, has a height of 8-10 km in polar regions, 10-12 km in temperate latitudes, and 16-18 km at the equator. Approximately 80-90% of the total mass of the atmosphere and nearly all of the water vapor are concentrated in the troposphere. As one ascends in the troposphere, the temperature decreases by an average of 0.65° per 100 m, eventually reaching 220 K (-53 °C) in the upper region. This upper region of the troposphere is referred to as the tropopause.
The stratosphere is a layer of the atmosphere that spans from 11 to 50 km above the Earth’s surface. It is distinguished by its relatively stable temperatures within the lower stratosphere layer (11-25 km) and its temperature increase within the upper stratosphere layer or inversion region (25-40 km), where temperatures rise from -56.5 to 0.8 °C. Once the altitude reaches around 40 km, the temperature remains constant at approximately 273 K (0 °C) until about 55 km. This level with a constant temperature is known as the stratopause and marks the boundary between the stratosphere and the mesosphere.
Most of the ultraviolet radiation in the range of 180-200 nm is captured and transformed in the stratosphere. The magnetic fields are altered, molecules break apart, ionization occurs, and new chemical compounds are formed under the influence of these rays. These phenomena can be observed as phenomena such as the Northern Lights and zarnitsa.
In the stratosphere and higher layers, gas molecules dissociate into atoms due to solar radiation (CO2 and H2 dissociate above 80 km, O2 dissociates above 150 km, and H2 dissociates above 300 km). In the ionosphere, which is located at altitudes of 100-400 km, gas ionization also takes place. At an altitude of 320 km, the concentration of charged particles (O+2, O-2, N+2) is approximately 1/300 of the concentration of neutral particles. Free radicals such as OH- and NO-2 are also present in the upper atmosphere.
There is hardly any moisture in the stratosphere.
The mesosphere commences at a height of 50 km and stretches to 80-90 km. The temperature of the air decreases to -88 °C until reaching a height of 75-85 km. The mesopause serves as the upper boundary of the mesosphere.
The thermosphere (also known as the ionosphere), the atmospheric layer that follows the mesosphere, starts at an altitude of 80-90 km and extends up to 800 km. The temperature of the air in the thermosphere rises rapidly and consistently, reaching several hundred or even thousand degrees.
The exosphere, which is the dispersal zone and the outer part of the thermosphere, is situated above 800 km. The gas in the exosphere is extremely rarefied, and its particles leak into interplanetary space from this region (dissipation).
Below a height of 100 km, the atmosphere is a uniform, well-mixed blend of gases. In the upper layers, the arrangement of gases by altitude depends on their molecular weights, and the concentration of heavier gases decreases more rapidly as you move away from the Earth’s surface. As the density of gases decreases, the temperature also decreases from 0 °C in the stratosphere to -110 °C in the mesosphere. However, at altitudes of 200-250 km, the individual particles have a kinetic energy that corresponds to a temperature of approximately 1500 °C. Above 200 km, there are significant fluctuations in temperature and gas density in both time and space.
At an altitude of approximately 2000-3000 km, the exosphere transitions gradually into the near-space void, known for being populated by sparsely distributed interplanetary gas particles, primarily hydrogen atoms. However, this gas constitutes merely a fraction of the interplanetary material present. The remaining portion encompasses dust-like particles originating from comets and meteors. This space is also permeated by electromagnetic and corpuscular radiation originating from both the sun and the galaxy.
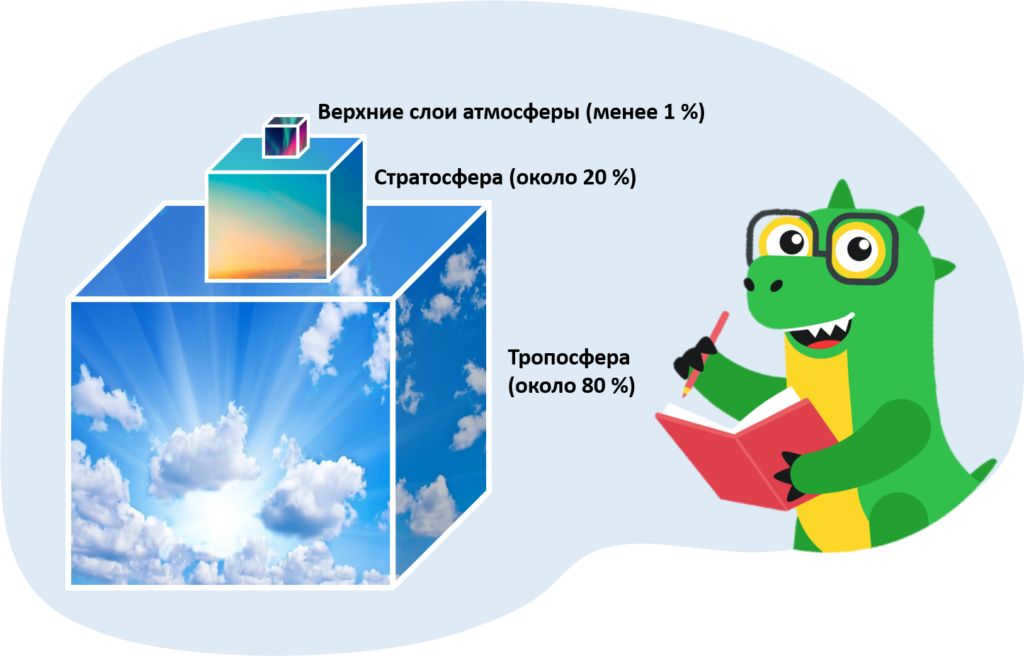
The atmosphere is composed of different layers, each with its own unique characteristics. The troposphere, which makes up about 80% of the atmosphere’s mass, is the layer closest to the Earth’s surface. Above it is the stratosphere, accounting for about 20% of the mass. The mesosphere, on the other hand, only makes up a small fraction of the atmosphere’s mass, around 0.3%. Lastly, the thermosphere accounts for less than 0.05% of the total mass.
When it comes to the electrical properties of the atmosphere, two distinct layers can be identified: the neutrosphere and the ionosphere. The neutrosphere refers to the neutral part of the atmosphere, while the ionosphere is where ions and free electrons are present. It is currently believed that the atmosphere extends up to a height of 2000-3000 km.
The composition of gases in the atmosphere also plays a role in its classification. The homosphere is the layer where gases are well mixed and homogeneous. In contrast, the heterosphere is the region where gravity influences the separation of gases, leading to a variable composition. The boundary between these two layers, known as the turbopause, is located at an altitude of approximately 120 km.
As we ascend in altitude, the total pressure of the atmosphere decreases, resulting in a decrease in the partial pressure of oxygen. Despite this, the atmosphere still provides us with the essential oxygen needed for breathing.
In the human lungs, there is a constant presence of approximately 3 liters of alveolar air. This alveolar air has a partial pressure of oxygen of 110 mm Hg, a carbon dioxide pressure of 40 mm Hg, and a water vapor pressure of 47 mm Hg at normal atmospheric pressure. As we go higher in altitude, the pressure of oxygen decreases, while the total pressure of water vapor and carbon dioxide in the lungs remains relatively constant at around 87 mmHg. When the ambient air pressure reaches this value, the supply of oxygen to the lungs will cease.
At approximately 19-20 km above the Earth’s surface, the atmospheric pressure decreases to 47 mmHg. As a result, water and interstitial fluid in the human body start to boil at this altitude. Death occurs almost immediately outside of a pressurized cabin at these altitudes. Therefore, from a human physiology standpoint, the “space” actually begins at an altitude of 15-19 km.
The dense layers of air, known as the troposphere and stratosphere, serve as a protective shield against harmful radiation. However, as the air becomes less dense at altitudes above 36 km, the organism is subjected to intense ionizing radiation from primary cosmic rays. Additionally, at altitudes above 40 km, the ultraviolet part of the solar spectrum, which poses a danger to humans, becomes active.
As we ascend to higher altitudes above the Earth’s surface, familiar phenomena observed in the lower atmosphere, such as sound propagation, aerodynamic lift and drag, and heat transfer by convection, gradually diminish and eventually disappear.
In the rarified layers of air, sound propagation becomes impossible. Up to heights of 60-90 km, it is still possible to utilize air resistance and lifting force for controlled aerodynamic flight. However, at altitudes of 100-130 km, concepts familiar to every pilot, such as the M “number” and the sound barrier, lose their significance. Despite this, at high flight speeds, it is still possible to employ an aerodynamic wing.
When the altitude reaches 180-200 km, the phase of purely ballistic flight commences, which can only be controlled through the use of jet forces. If the flight generates a centrifugal force that matches the force of gravity at a specific altitude, the aircraft transforms into an artificial satellite of the Earth.
At altitudes exceeding 100 km, the atmosphere loses another significant property – the ability to absorb, conduct, and transfer heat energy through convection (i.e., air mixing). Consequently, various components and equipment of the orbital space station cannot be cooled externally, as is typically accomplished in an airplane using air jets and radiators. In such altitudes, as well as in space in general, the sole means of heat transfer is thermal radiation.
Wow! The experienced astronomy enthusiast can’t believe it when he comes across this headline. He knows that such an event is not expected to happen anytime soon. That’s correct. Not in the foreseeable future. However, in tens of thousands of years, our distant and forgotten descendants from the 21st century will have the opportunity to witness such a phenomenon. Little will they know that over 60,000 years ago, people already had knowledge of July 26.
All whole numbers (except 0 and 1) have at least two factors: 1 and the number itself. Numbers that have no other factors are known as prime numbers. Numbers that have additional factors are known as composite (or complex) numbers. There are an infinite amount of prime numbers. The following are prime numbers that are less than or equal to 200: 2, 3, 5, 7, 11, 13, 17, 19, 19, 23, 29, 31, 37, 41, 43, 47, 53, 53, 59, 61, 67, 71, 73, 73, 79, 83, 89, 97, 101, 103.
Take this test to assess your child’s understanding of school and school routines. Have your child answer the questions below. Each correct answer earns one point.
1. Define a classroom. (A classroom is a designated space where classes are conducted. It typically contains desks, a teacher’s desk, and a chalkboard for assignments and exercises. Additionally, it refers to the group of students who attend the class together.)
The eminent Russian critic V. G. Belinsky posited that poetry’s purpose is “to extract the poetry of life from the prose of life and to shock the soul with a faithful depiction of life.” An author who accomplishes this task, who manages to stir the soul with portrayals of even the most mundane aspects of human existence, is N.V. Gogol. In my view, Gogol’s greatest contribution to Russian society is .
Our planet retains its air thanks to the gravitational force exerted by the Earth. As a cohesive entity, the atmosphere moves in sync with the Earth’s rotation.

The atmosphere is bounded by the Earth’s surface at its lower limit and is commonly considered to extend to a height of 1000-1200 km. As altitude increases, the air becomes less dense and gradually transitions into the vast expanse of interplanetary space.
Meteorology (derived from the Greek words “meteora” meaning “celestial” and “logos” meaning “study”) is a scientific discipline dedicated to the investigation of the atmosphere and its associated processes.
To study the atmosphere, various tools and vehicles are utilized, including research vessels, aircraft, radiosondes, artificial satellites, and spacecraft.


The Earth’s atmosphere was created in what way?
According to some experts, the initial atmosphere of the Earth was formed in space and consisted mainly of helium and hydrogen. The secondary atmosphere was enriched with carbon dioxide due to volcanic activity. This led to the formation of clouds and heavy rainfall. As a result, the hydrosphere was established and green plants emerged, absorbing carbon dioxide and releasing oxygen.
The Structure of the Atmosphere
Other planets in our solar system also possess an atmosphere. However, what sets Earth’s atmosphere apart is its distinctive blend of gases, which played a crucial role in the emergence of life on our planet.
The atmosphere is comprised of a diverse array of gases, along with water droplets, ice crystals, dust particles, soot, and organic substances.
The primary constituents of the atmosphere consist of nitrogen, comprising approximately 78% of the air, oxygen, which accounts for about 21%, and carbon dioxide, making up a mere 0.038%. The presence of noble gases in the atmosphere is relatively minimal, constituting less than 1%. These noble gases include argon, composing 0.93%, as well as krypton, xenon, neon, helium, hydrogen, and ozone.
At an altitude of 12 km, the air maintains a relatively consistent composition. However, as we ascend higher, the proportion of lighter gases such as hydrogen and helium becomes more prevalent.
Composition of the Atmosphere
The atmosphere is divided into three primary layers: the troposphere, stratosphere, and upper layers (mesosphere, thermosphere, ionosphere, exosphere). Each layer possesses distinct properties, with variations in air density and temperature being particularly noteworthy.
The majority of Earth’s air is found in the lower layers of the atmosphere, specifically the troposphere and stratosphere.
Troposphere
The troposphere, which gets its name from the Latin word “tropos” meaning “turn” or “change”, is the lowest and most densely packed layer of air surrounding the Earth.
About 80% of the total mass of air and nearly all of the water vapor is found within the troposphere.
The thickness of the troposphere varies depending on the latitude. In polar regions, the troposphere measures 8-9 km in thickness, while in temperate latitudes it is 10-12 km thick. In the tropics and at the equator, the troposphere can reach a thickness of 16-18 km. The equator experiences the rise of warm air to high altitudes, while the cold air at the poles is unable to ascend to such heights.
The troposphere is responsible for both horizontal and vertical air mixing. It is here that air masses, clouds, cyclones, and anticyclones form, giving rise to hurricanes and tornadoes. Due to its role in weather formation, the troposphere is often referred to as the “weather factory.”
As one ascends through the troposphere, the temperature gradually decreases, reaching -50…-55 ° C at its upper boundary.
The troposphere is also the crucial layer supporting life. It is home to humans, animals, and plants.
The stratosphere, derived from the Latin word “stratum” meaning “planking” or “layer”, is the Earth’s second atmospheric layer.
The stratosphere spans from approximately 50-55 km above the Earth’s surface. It encompasses about 20% of the Earth’s total air mass. Due to the thinness of the air, it is not possible to breathe in the stratosphere.
Clouds are infrequent in the stratosphere due to the low water vapor content. Strong winds are a characteristic feature of this layer.
The temperature in the lower part of the stratosphere remains relatively constant. However, as you ascend to heights above 25 km, the temperature gradually rises, eventually reaching almost 0°C at the upper boundary of the stratosphere.
Ozone layer
The ozone layer is situated in the stratosphere, specifically at an elevation of 20 to 40 km (25-30 km in tropical latitudes, 20-25 km in temperate latitudes, and 15-20 km in polar latitudes).
The ozone layer, also known as the ozone shield, has a vital function in safeguarding life on Earth by shielding living organisms from harmful ultraviolet radiation.
What causes ozone holes and how can they impact human health?
An ozone hole is a region within the ozone layer where harmful solar rays can penetrate.
Ozone holes form when there is a decrease in ozone concentrations, with the largest ones occurring over Antarctica.
There are numerous factors contributing to the formation of ozone holes, but the primary cause is human pollution. Combustion byproducts released into the atmosphere from factories and plants lead to the destruction of ozone molecules.
Additionally, jet airplanes, which emit substances harmful to the ozone layer through their engines, also contribute to the creation of ozone holes. Nuclear tests also have a significant impact on the ozone layer.
Another factor in the destruction of the ozone layer is the use of mineral fertilizers, which react with soil bacteria when applied to the ground.
The depletion of the ozone layer has detrimental effects on human well-being. The level of ozone in the atmosphere is declining, leading to an increase in the amount of solar radiation that reaches the Earth’s surface and contributes to the development of skin cancer.
The higher regions of the atmosphere
Located above 50-55 km, the upper regions of the atmosphere encompass the mesosphere, thermosphere, ionosphere, and exosphere.
In the layer between the stratosphere and the thermosphere (derived from the Greek word “meso” meaning “middle”), the air density is 200 times lower than at the Earth’s surface. It is in this region that the highest clouds, known as silvery clouds, are formed. The thermosphere (derived from the Greek word “thermos” meaning “warm”) is the hottest layer of the atmosphere. Temperatures in this layer can range from +500°C to +1500°C. The exosphere (derived from the Greek word “exo” meaning “outside”) is the outermost layer of the atmosphere. It is from this layer that hydrogen, oxygen, and helium, which can overcome the Earth’s gravity, escape into outer space. The ionosphere refers to the outer layer of the atmosphere with a high concentration of free ions and electrons. This layer encompasses both the thermosphere and the exosphere.

The Significance of the Atmosphere
Out of all the planets in the solar system, Earth’s atmosphere stands out as the only air envelope that contains the essential oxygen required for breathing. Additionally, it also holds the necessary amount of carbon dioxide needed for plant life to produce organic matter. Nitrogen plays a crucial role in maintaining a stable oxygen content and is also essential for plant nutrition.
Moreover, the atmosphere acts as a protective shield against harmful cosmic rays and small meteorites, which burn up in its upper layers.
Functioning as a massive blanket, the atmosphere safeguards the planet from excessive cooling at night and excessive heating during the day.
The lower layers of the atmosphere serve as a “weather factory,” where all weather phenomena originate.

Radio waves are able to travel through the earth’s atmosphere, which allows for communication to take place. The atmosphere plays a crucial role in the Global Water Cycle.
Frequently Asked Questions
The earth’s gravitational force is what keeps the air in our atmosphere. As the earth rotates, the atmosphere moves along with it.
The earth’s atmospheric air is made up of a mixture of different gases, including nitrogen (78%), oxygen (21%), argon (0.93%), carbon dioxide (0.038%), and other gases such as krypton, xenon, neon, helium, hydrogen, and ozone.
The atmosphere is divided into three main layers: the troposphere, the stratosphere, and the upper layers (mesosphere, thermosphere, ionosphere, and exosphere). Each layer has its own unique properties, including air density and temperature.
The air gets heated as the sun’s rays fall on the surface of the earth. As we move away from the surface, the heating becomes weaker, resulting in a decrease in temperature in the troposphere. On the other hand, the temperature increases in the stratosphere due to the presence of a high ozone content at an altitude of 20-25 km, which forms the ozone layer.
The troposphere is the most suitable layer for life due to its air density, composition, temperature, humidity, and other characteristics.
In the troposphere, air masses and clouds form, leading to precipitation and the occurrence of weather phenomena such as hurricanes and tornadoes.
The Earth is surrounded by a layer of gas, primarily nitrogen with a high oxygen content and trace amounts of other molecules.

The Earth’s atmosphere is surrounded by a layer of gases that scatter blue light, giving it a visible blue appearance when observed from the International Space Station (ISS) at an altitude of 335 kilometers (208 miles) [1].
When considering the composition of the Earth’s atmosphere by volume, excluding water vapor, trace gases play a significant role. These trace gases make up approximately 0.04333391% of the atmosphere, with a concentration of 0.04402961% as of April 2019 [2] [3]. The data provided in the bottom chart is primarily from 2000, with the exception of CO2 and methane, which are from 2019. It’s important to note that these figures do not represent any specific source [4].
Within the Earth’s atmosphere, a layer of gases, commonly referred to as the air, is held in place by the planet’s gravity, encircling the globe and shaping its planetary atmosphere. The Earth’s atmosphere plays a crucial role in safeguarding life on this planet, as it generates pressure that permits the existence of liquid water on the surface, absorbs solar radiation, particularly ultraviolet rays, warms the surface by retaining heat (known as the greenhouse effect), and lessens temperature disparities between day and night (during diurnal temperature fluctuations).
Dry air is composed of various gases, with nitrogen making up 78.09% of its volume, oxygen accounting for 20.95%, argon comprising 0.93%, carbon dioxide contributing 0.04%, and trace amounts of other gases present as well. [8] Additionally, air contains different levels of water vapor, typically averaging around 1% at sea level and 0.4% throughout the entire atmosphere. The composition of air, as well as its temperature and atmospheric pressure, varies depending on altitude. This unique combination of gases and conditions makes air suitable for supporting photosynthesis in terrestrial plants and respiration in terrestrial animals, and it is only found in the troposphere of Earth’s natural environment and in artificial atmospheres.
The total mass of the Earth’s atmosphere is approximately 5.15 × 10 18 kilograms, with around three quarters of that concentrated within 11 kilometers (6.8 miles; 36,000 feet) of the Earth’s surface. As one ascends higher into the atmosphere, it gradually becomes thinner and thinner, with no precise boundary separating it from the expanse of outer space. For the purposes of reference, the Karman Line is often employed as a delineation point, situated at an altitude of 100 kilometers (62 miles) above the Earth’s surface, which is about 1.57% of the Earth’s radius. When spacecraft undergo re-entry into the Earth’s atmosphere, noticeable atmospheric effects begin to manifest at an altitude of roughly 120 kilometers (75 miles). The atmosphere itself can be divided into distinct layers, each characterized by unique features such as temperature and composition.
Atmospheric science, also known as aerology, is the study of Earth’s atmosphere and its processes. It encompasses various subfields, including climatology and atmospheric physics. Notable figures in the early development of this discipline include Leon Teysseren de Bort and Richard Assmann. The investigation of the historical atmosphere is referred to as paleoclimatology.
- 1 Essay
- 2 Stratification
- 2.1 Exosphere
- 2.2 Thermosphere
- 2.3 Mesosphere
- 2.4 Stratosphere
- 2.5 Troposphere
- 2.6 Other layers
- 3.1 Pressure and thickness
- 3.2 Temperature and speed of sound
- 3.3 Density and mass
- 4.1 Scattering
- 4.2 Absorption
- 4.3 Emission
- 4.4 Refractive index
- 6.1 Earliest atmosphere
- 6.2 The Second Atmosphere
- 6.3 The Third Atmosphere
- 6.4 Air Pollution
Essay
(A) The mole fraction is equal to the volume fraction only for an ideal gas. You can also refer to the volume in thermodynamics.
(B) ppmv: parts per million by volume.
(C) The concentration of CO2 has been increasing in recent decades.
(D) Water vapor accounts for about 0.25% of the total atmosphere by mass.
(E) The amount of water vapor varies significantly from one place to another [11].
The average molecular weight of dry air, which can be used to calculate density or convert mole fraction to mass fraction, is approximately 28.946 [14] or 28.96 [15] g/mole. This value decreases when the air is humid.
The relative concentration of gases remains constant up to an altitude of about 10,000 m (33,000 ft) [16].
The volume fraction of the main constituents of Earth’s atmosphere as a function of altitude is shown in the MSIS-E-90 atmospheric model.
Stratification
The Earth’s atmosphere is divided into four lower layers when viewed diagonally from the top of the exobase. These layers are depicted to scale, although the objects within them are not. It is important to note that the auroras, which are shown here at the bottom of the thermosphere, can actually form at any altitude within this particular layer of the atmosphere.
Typically, as you ascend in the atmosphere, air pressure and density decrease. However, the relationship between temperature and altitude is more complex. In certain regions, temperature can remain relatively constant or even increase with altitude. This behavior can be seen in the temperature section below. By analyzing balloon soundings, scientists can measure the overall pattern of temperature and use it as an indicator to distinguish different atmospheric layers. Consequently, the Earth’s atmosphere can be divided into five main layers, known as atmospheric stratification. With the exception of the exosphere, the atmosphere consists of four main layers: the troposphere, stratosphere, mesosphere, and thermosphere. [17] The five major layers, listed from highest to lowest, are:
- Exosphere: 700 to 10,000 kilometers (440 to 6,200 miles)
- Thermosphere: 80 to 700 km (50 to 440 miles) [18]
- Stratosphere: 12 to 50 km (7 to 31 miles)
- Troposphere: 0 to 12 km (0 to 7 miles) [19]
Exosphere
The exosphere is the outermost layer of Earth’s atmosphere, which is the upper boundary of the atmosphere. It stretches from the exobase layer, situated at the top of the thermosphere approximately 700 km above sea level, to around 10,000 km (6,200 mi; 33,000,000 ft), where it blends with the solar wind.
This layer primarily consists of very low concentrations of hydrogen, helium, and a few other heavier molecules, such as nitrogen, oxygen, and carbon dioxide, which are closer to the exobase. The atoms and molecules are so widely spaced that they can travel hundreds of kilometers without colliding with one another. As a result, the exosphere no longer behaves like a gas, and particles are constantly escaping into space. These freely moving particles follow ballistic trajectories and can migrate in and out of the magnetosphere or solar wind.
The exosphere is located too far above the Earth for any meteorological phenomena to occur. However, the Northern Lights and aurora australis occasionally take place in the lower part of the exosphere, where they overlap with the thermosphere. The exosphere is home to the majority of the Earth’s satellites.
Thermosphere
The thermosphere, Earth’s atmosphere’s second highest layer, spans from the mesopause (which separates it from the mesosphere) at approximately 80 km (50 miles; 260,000 feet) in altitude to the thermopause, located between 500-1000 km (310-620 miles; 1,600,000-3,300,000 feet) above the Earth’s surface. The height of the thermopause varies significantly due to fluctuations in solar activity. [18] This region is also referred to as the exobase since it marks the lower boundary of the exosphere. Within the lower section of the thermosphere, ranging from 80 to 550 kilometers (50 to 342 miles) above the Earth’s surface, lies the ionosphere.
The temperature of the thermosphere gradually increases as you go higher, reaching as high as 1,500 °C (2,700 °F). However, because the gas molecules are so spread out, this temperature doesn’t have much meaning in everyday terms. The air in the thermosphere is so thin that individual molecules, like oxygen, travel about 1 kilometer (0.62 miles; 3,300 feet) on average before colliding with another molecule. [20] Despite having many high-energy molecules, the thermosphere wouldn’t feel hot to humans on direct contact because its density is too low to transfer a significant amount of energy to or from the skin.
The thermosphere is a cloudless layer that is devoid of water vapor. Nevertheless, it occasionally offers sightings of non-hydrometeorological events like the Northern Lights and aurora australis. The International Space Station, orbiting at altitudes between 350 and 420 kilometers (220 and 260 miles), resides within this atmospheric layer. Additionally, the majority of Earth’s satellites are situated in this region.
The mesosphere is the third highest layer of Earth’s atmosphere, located between the stratosphere and the thermosphere. It spans from the stratopause, which is approximately 50 km (31 mi; 160,000 ft) above sea level, to the mesopause at 80-85 km (50-53 mi; 260,000-280,000 ft) above sea level.
As altitude increases, the temperature gradually decreases until reaching the mesopause, which marks the upper boundary of this intermediate atmospheric layer. It is the coldest region on Earth, with an average temperature of around -85.° C (-120 ° F; 190 K). [21] [22]
Directly below the mesopause, the temperature drops so significantly that even a minimal amount of water vapor at this altitude can transform into the polar-mesospheric environment, creating silver clouds. These clouds are the highest in the atmosphere and can be observed with the naked eye when sunlight bounces off them approximately one to two hours after sunset or before sunrise. Their visibility is greatest when the Sun is situated 4 to 16 degrees below the horizon. Transient light events (TLEs), which are lightning-induced discharges, occasionally form in the mesosphere above thunderclouds in the troposphere. Additionally, the mesosphere serves as the layer where the majority of meteors burn up upon entry into the atmosphere. It is situated at an elevation that is too high for jet airplanes and balloons to access, yet too low to accommodate orbiting spacecraft. Sounding rockets and rocket aircraft are primarily employed to explore and study the mesosphere.
Stratosphere
The stratosphere is the second highest layer of the Earth’s atmosphere. It is situated above the troposphere and is distinguished from it by the presence of the tropopause. The stratosphere starts approximately 12 km (7.5 mi; 39,000 ft) above the Earth’s surface and extends up to the stratopause at an elevation of 50 to 55 km (31 to 34 mi; 164,000 to 180,000 ft).
The pressure at the top of the stratosphere is approximately 1/1000 of the pressure at sea level. It contains the ozone layer, which is a region in Earth’s atmosphere that has higher concentrations of this gas. The stratosphere is a layer where the temperature increases as altitude increases. This temperature increase is a result of the ozone layer absorbing ultraviolet (UV) radiation from the Sun, which reduces turbulence and mixing. While the temperature can be as low as -60 °C (-76 °F; 210 K) in the tropopause, the upper stratosphere is much warmer and can reach around 0 °C. [23]
The temperature profile of the stratosphere creates a remarkably stable atmosphere, devoid of the air turbulence that characterizes the troposphere and induces weather conditions. As a result, the stratosphere is mostly cloud-free and unaffected by other weather phenomena. Nevertheless, in the lower portion of this atmospheric layer, where the air is coldest, polar stratosphere or nacreous clouds can occasionally be observed. It is worth noting that the stratosphere represents the uppermost altitude that a jet airplane can reach.
The troposphere, which is the lowest layer of Earth’s atmosphere, spans from the Earth’s surface up to an average height of approximately 12 km (7.5 mi; 39,000 ft). However, this altitude can vary from around 9 km (5.6 mi; 30,000 ft) at the geographic poles to 17 km (11 mi; 56,000 ft) at the Equator, with some fluctuations caused by weather patterns. Looking down from above, the troposphere is enclosed by the tropopause, a boundary that is typically characterized by a temperature inversion (meaning a layer of comparatively warm air sitting atop cooler air). In certain areas, the tropopause is also defined by a zone where the temperature remains constant as altitude changes.
While there may be variations, the temperature in the troposphere generally decreases as altitude increases due to the fact that the troposphere is primarily heated by energy transfer from the surface. As a result, the lowest part of the troposphere (also known as the Earth’s surface) tends to be the warmest. This promotes vertical mixing, which is why it is called the troposphere, derived from the Greek word “tropos” meaning “turn”. The troposphere contains about 80% of the Earth’s atmosphere’s mass. [26] It is denser than the layers above it because the weight of the atmosphere is greatest at the top of the troposphere, causing it to compress more strongly. Approximately 50% of the atmosphere’s total mass is found within the lower 5.6 km (18,000 feet) of the troposphere.
The troposphere contains the majority of atmospheric water vapor or moisture, making it the primary layer for weather phenomena on Earth. It is characterized by various cloud types formed by wind circulation, although exceptionally tall cumulonimbus thunderstorm clouds can reach the lower stratosphere by breaking through the tropopause. The troposphere is also the main area for aviation operations, with propeller aircraft being limited to this layer.

The Space Shuttle endeavor can be seen orbiting in the thermosphere in this image. Due to the perspective, it gives the illusion of covering the stratosphere and mesosphere, which are actually situated more than 250 kilometers (160 miles) below. The orange layer represents the troposphere, followed by the whitish stratosphere and then the blue mesosphere. [27]
Among the five primary layers outlined above, which are primarily determined by temperature, there are several secondary layers that differ in various properties:
- The stratosphere contains the ozone layer. Within this layer, ozone concentrations range from 2 to 8 parts per million, which is significantly higher than in the lower atmosphere, but still relatively low compared to the main components of the atmosphere. The ozone layer is mainly situated in the lower stratosphere, approximately 15-35 km (9.3-21.7 mi; 49,000-115,000 ft) above the Earth’s surface. However, its thickness can vary depending on the season and geographic location. Approximately 90% of the ozone in Earth’s atmosphere is concentrated in the stratosphere.
- The ionosphere, which is ionized by solar radiation, is a region of the atmosphere that is responsible for the occurrence of auroras. During the day, it spans from 50 to 1,000 km (31 to 621 miles, 160,000 to 3,280,000 feet) and includes the mesosphere, thermosphere, and parts of the exosphere. However, the ionization in the mesosphere mostly stops at night, so the polar lights are generally seen only in the thermosphere and lower exosphere. Additionally, the ionosphere acts as the inner border of the magnetosphere, which is practically significant as it impacts the propagation of radio waves on Earth.
- Whether the atmospheric gases are well mixed determines the division of the atmosphere into the homosphere and heterosphere. The homosphere, which includes the troposphere, stratosphere, mesosphere, and lower part of the thermosphere, is characterized by a uniform chemical composition due to the mixing of gases by turbulence. This uniform layer extends up to the turbopause, located at approximately 100 km (62 mi; 330,000 ft), which is considered the edge of space by the FAI and is about 20 km (12 mi; 66,000 ft) above the mesopause.