Dear readers, technicians, and humanitarians, I urge you to take immediate action and step outside to bask in the warm summer sun (if the weather permits). This is not a drill! I repeat, this is not a drill! If those around you don’t understand the urgency of the situation, then please make yourself comfortable and let’s discuss the topic of lighting. In a nutshell, this article focuses on the impact of indoor lighting on our bodies. I’ll try not to overwhelm you with technical details, but I’ll provide appropriate links for those who are interested. However, I can’t resist including some graphs (I simply love them). Since this article is quite lengthy, I’ve decided to start by examining the spectral characteristics of illumination (you can find more details here).
So, just picture, my friends, a scenario where there resides an ordinary individual somewhere around the globe, let’s give him the name Vasily. Vasily spent 20 years dwelling on the outskirts of the forest in the mid-region of our vast homeland, but he yearned for “top-notch barista-made coffee,” trendy sweatshirts, and glossy iPhones, so he made up his mind to venture into the city. And to make matters worse, he decided to take up a job in the basement floor office of the illustrious city of Murmansk, or rather, in the “concrete cubicle farm,” where he would toil tirelessly and be deprived of sunlight.
And behold, this is what awaited Vasily there, concealed beneath the surface, a curiosity for all inquisitive minds to uncover.
The article will have a considerable size and will be divided into three sections in terms of content.
1 – The spectral properties of light sources
2 – Ways to measure the spectrum using simple tools and “blue tape”
3 – A brief overview of the impact of light on human beings


*Please note that the spectra shown in this article may differ from the actual light sources due to technical limitations. If you want to verify it yourself.
Part 1 – Spectral Characteristics of Light Sources
First, let’s examine the key aspects.
1. Prior to the widespread adoption of electric lighting in households, humanity primarily adapted its daily activities to natural light.
2. Natural light varies throughout the day and spans a continuous spectrum of radiation, including ultraviolet, visible, and infrared wavelengths. Unlike artificial lighting, natural light does not exhibit pulsation.
3. In modern times, individuals typically spend a significant portion (approximately 90%) of their time indoors, which can be considered an artificial environment.
4. Indoors, people often rely on artificial lighting, either exclusively or in combination with natural light. Even during the summer, not everyone has the opportunity to rely solely on natural light.
5. Light has a direct impact on various biological processes within the human body.
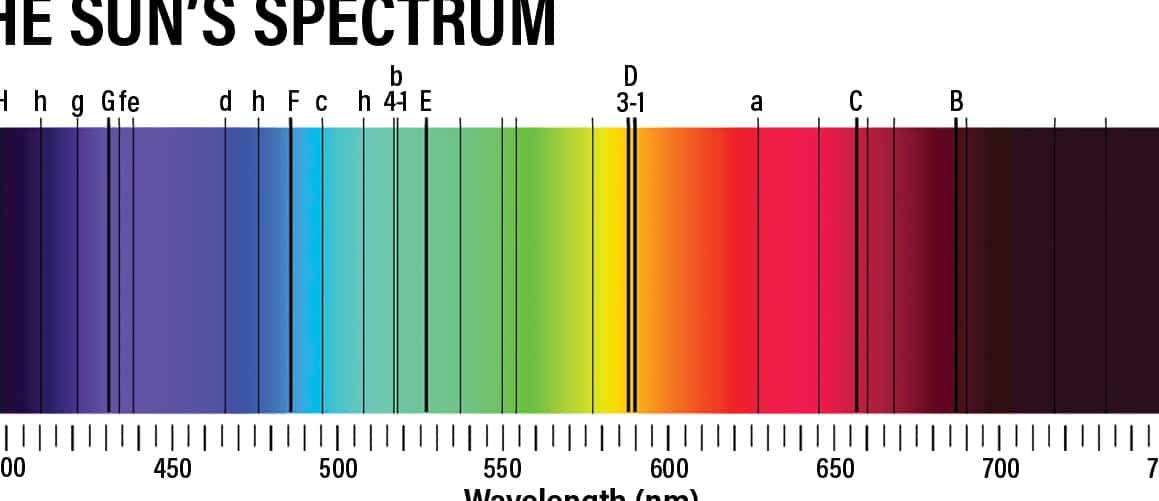
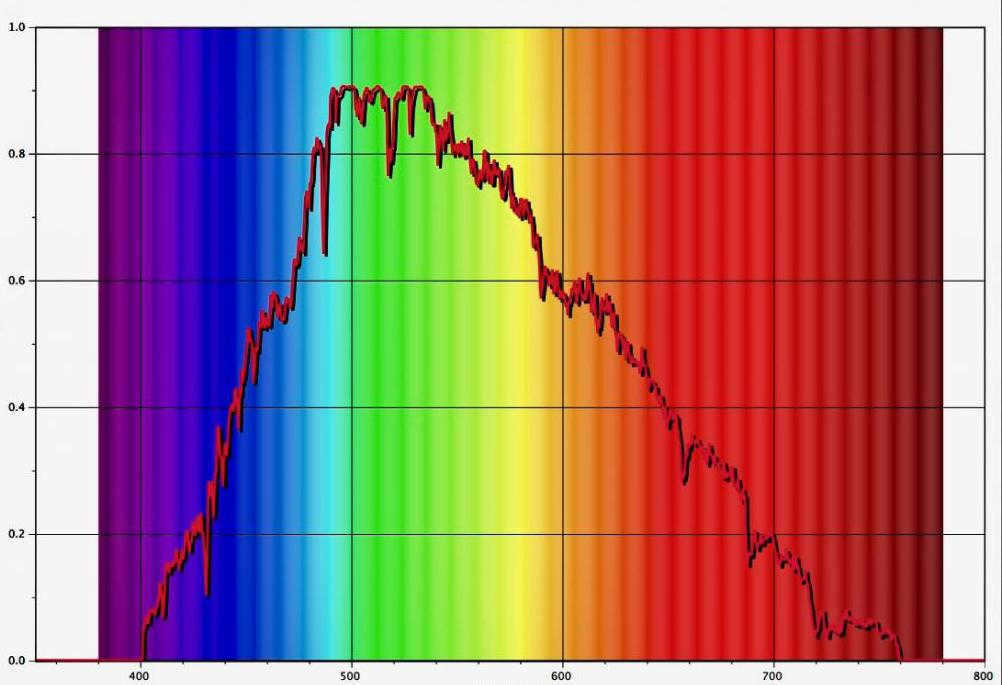
Here is an image of the sun’s spectrum, featuring the “rainbow” and a graph. It appears that someone diligently captured the sky in Moscow.

Let’s return to Vasily. As we recall, he spent most of his conscious life in nature. Now, let’s examine how the sunlight illuminated him and why hedgehogs developed thorns more quickly.
A group of responsible individuals, who specialize in lighting technology, have created a model of artificial daylight with varying color temperatures (in the form of an xls file that allows simulation, so don’t worry). Let’s imagine that in the early morning, Vasily was bathed in sunshine with a temperature of 4000K. At noon, the temperature increased to 5500K, and throughout the day, it reached 7000K. Then, as night fell, the temperature gradually decreased in reverse order (you can find approximate color temperatures of light sources here).
However, a beautiful sun like that shone a long time ago, what awaits our protagonist trapped in a “concrete box”?
Given that the majority of individuals who work in non-manufacturing positions are unlikely to be confined to offices of the highest grade, there are many individuals (including myself) who are eagerly anticipating it.

Affordable fluorescent bulbs featuring electromagnetic start and control mechanisms, such as the LB-40 model, offer a high color rendering index (CRI), indicating their ability to accurately reproduce colors.
Perhaps more expensive options can be found abroad. However, it’s worth mentioning compact fluorescent lamps with a CRI exceeding 80, as I have a chart that showcases their performance.
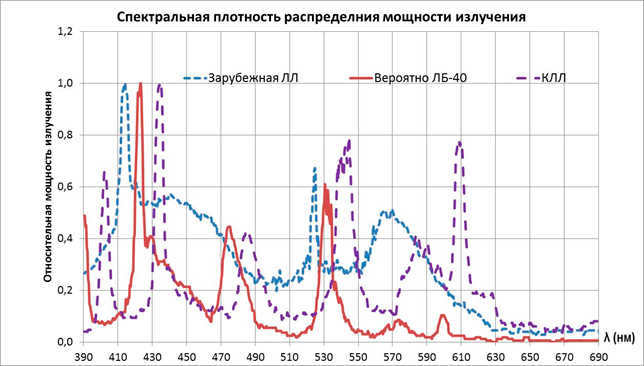
I suggest taking a look at pictures with the “rainbow” under the splayer and comparing them with the Moscow sky shown above. The difference will be quite apparent.
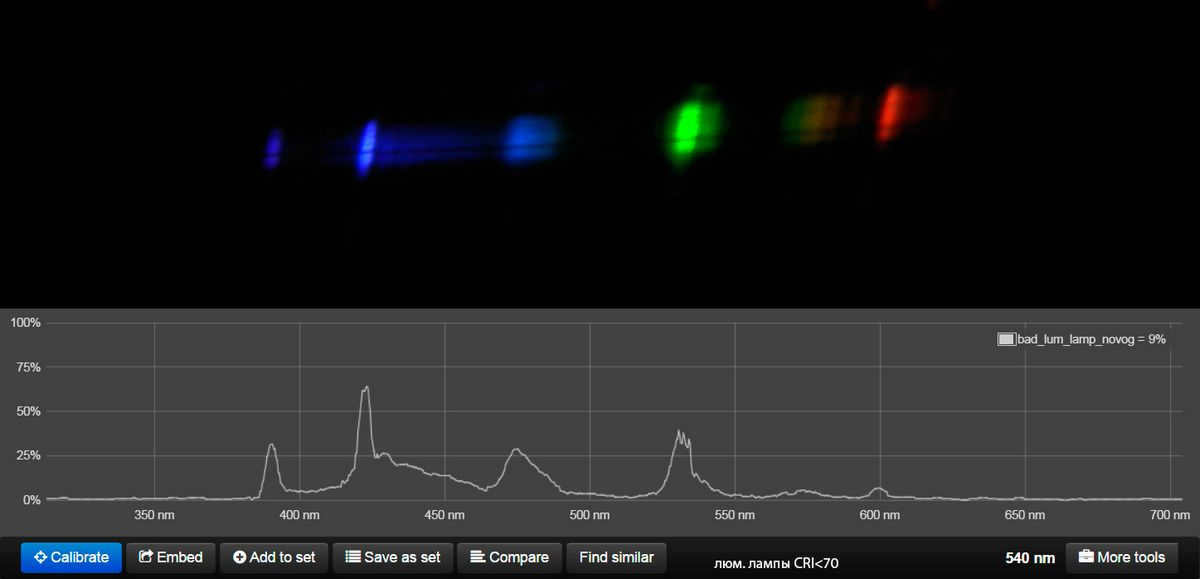
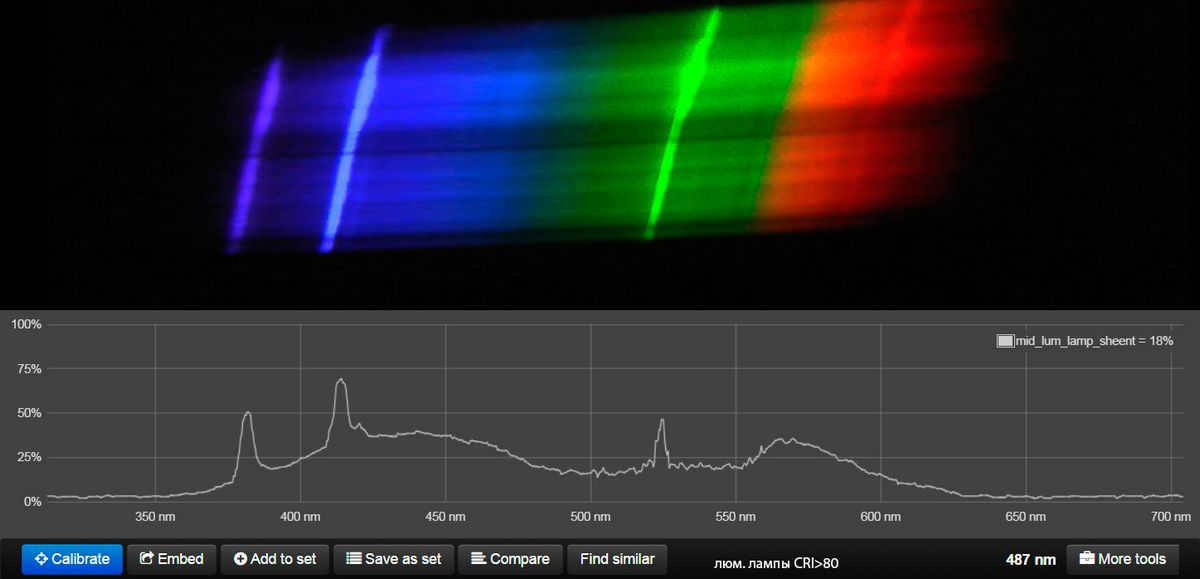
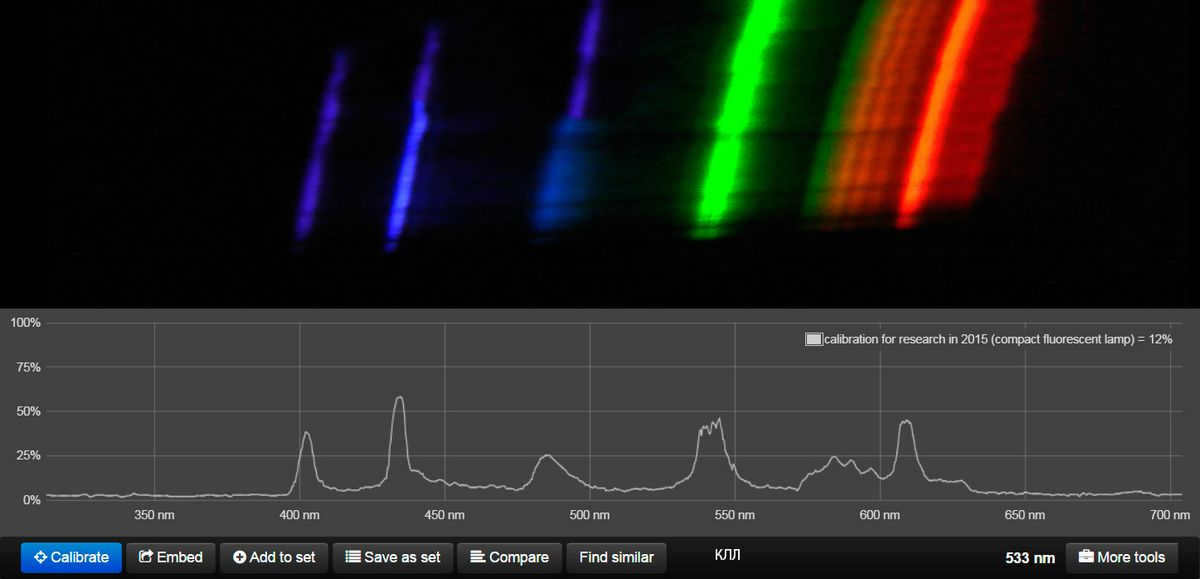



So, what we witness is the ultimate dream of every climber and a way to put friends to the test, just like V. Vysotsky did. We are talking about mountains and peaks, and the more affordable the lamp is, the more “Himalayas” we can explore.
What information does this provide us? This information provides us with the initial understanding that the light being discussed is not the same as natural light. Additionally, it highlights the fact that our test subject, Vasily, is required to remain under unchanged light for the entirety of his 8-hour workday. Remember the previously mentioned graph? Natural light undergoes changes throughout the day, however, the light Vasily is exposed to remains constant. As a result, our bodies suffer from the effects of being exposed to abnormal light, which is known to be linked to negative health outcomes, reduced visual performance, and decreased productivity. Need further proof? Just consult the esteemed Julian Borisovich Eisenberg, as cited in the Handbook of Lighting Engineering on page 889.

Is the solution to use LED lighting?
Well, maybe not exactly. However, it is already much better.
We examine the graphs and still see that “your spectrum doesn’t resemble mom’s at all”. There is still a peak in the blue region, a dip in the blue, and I must also remind you that most LED lamps emit only one color during the day.
I don’t have pictures for RGB LEDs, but trust me, the situation is not any better (and perhaps even worse) there.
I conducted measurements with what I had at hand 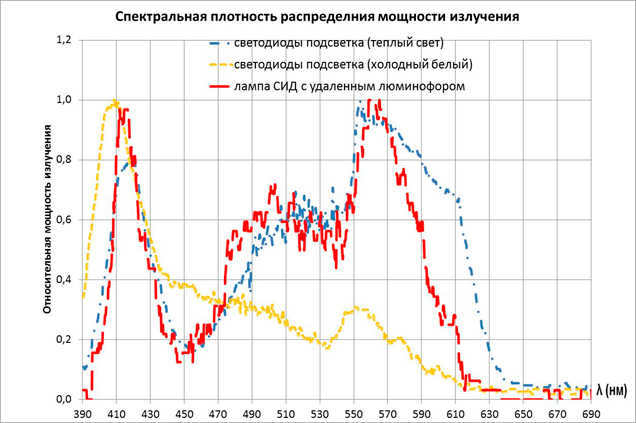
picture from the Internet 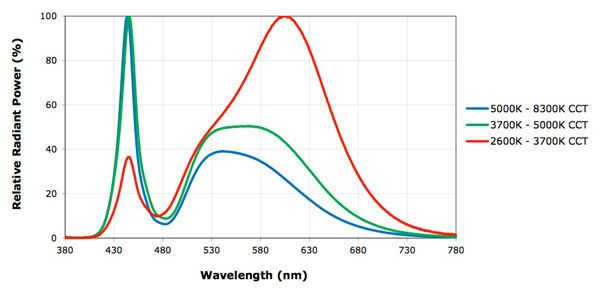
The spectrum of warm white LED is shown below the spoiler.
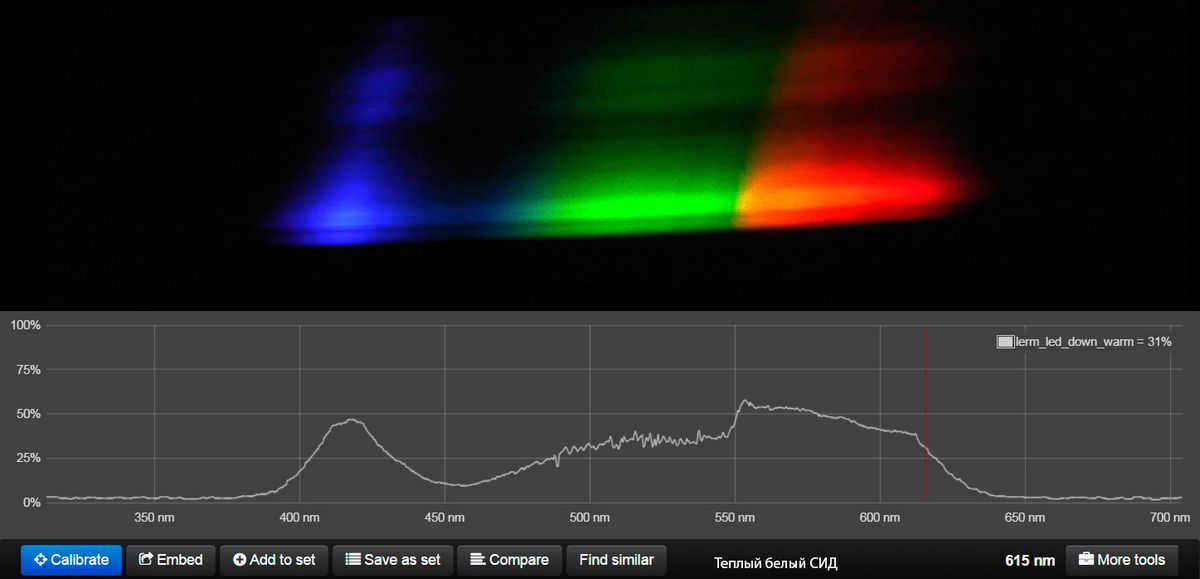
Therefore, after completing his 8-hour work shift, Vasily returns home, feeling tired of the artificial lighting at his workplace. As he enters his house, he takes a seat on the sofa and basks in the comforting glow of a warm lamp.
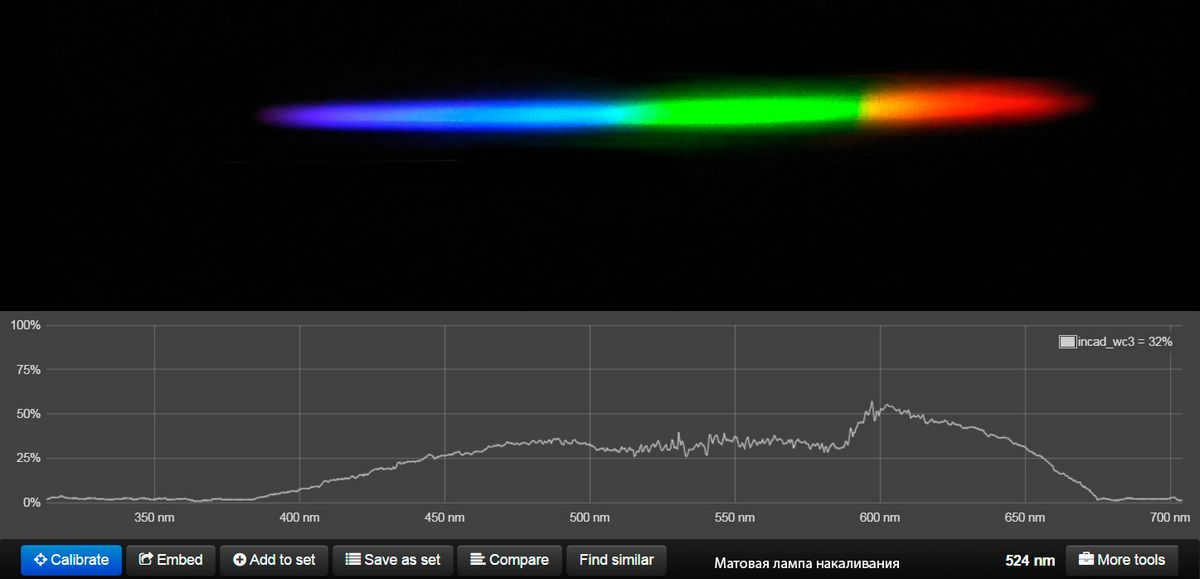
Interestingly enough, using an incandescent lamp for evening home lighting isn’t such a bad idea. Its spectrum closely resembles that of the evening sun, which means it doesn’t significantly hinder the production of melatonin (more on that later). The only drawback is that it isn’t adjustable throughout the day.
Below is the spectrum of an incandescent lamp:
Vassili was in his home, contemplating, pondering, and ultimately concluding that he would no longer compromise his well-being. Instead, he resolved to become a freelancer and take charge of the white light as he saw fit, all while working from the comfort of his own abode.
Interestingly enough, this option isn’t the worst, even though contemporary adjustable LED lamps still fall short of replicating natural lighting perfectly. Nevertheless, they still outperform the aforementioned LB-40 and may even surpass regular LED lamps to some extent. While RGBW lamps may be more of an experimentation, LED-based lamps that emit warm white and cool white light are quite suitable for illumination. If you’re intrigued, you can explore this avenue further.
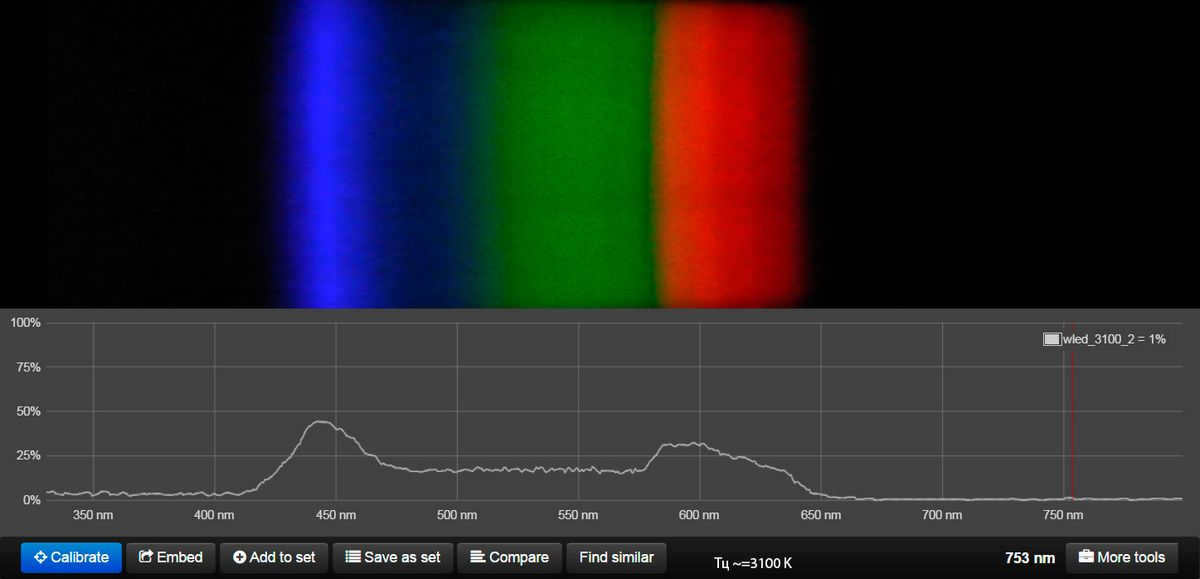
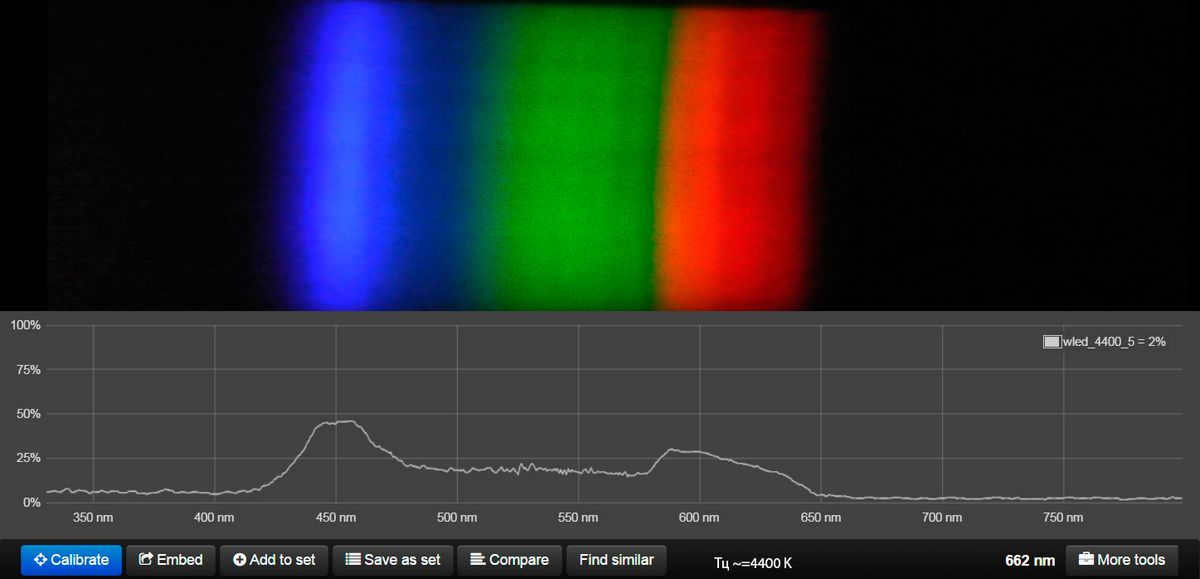
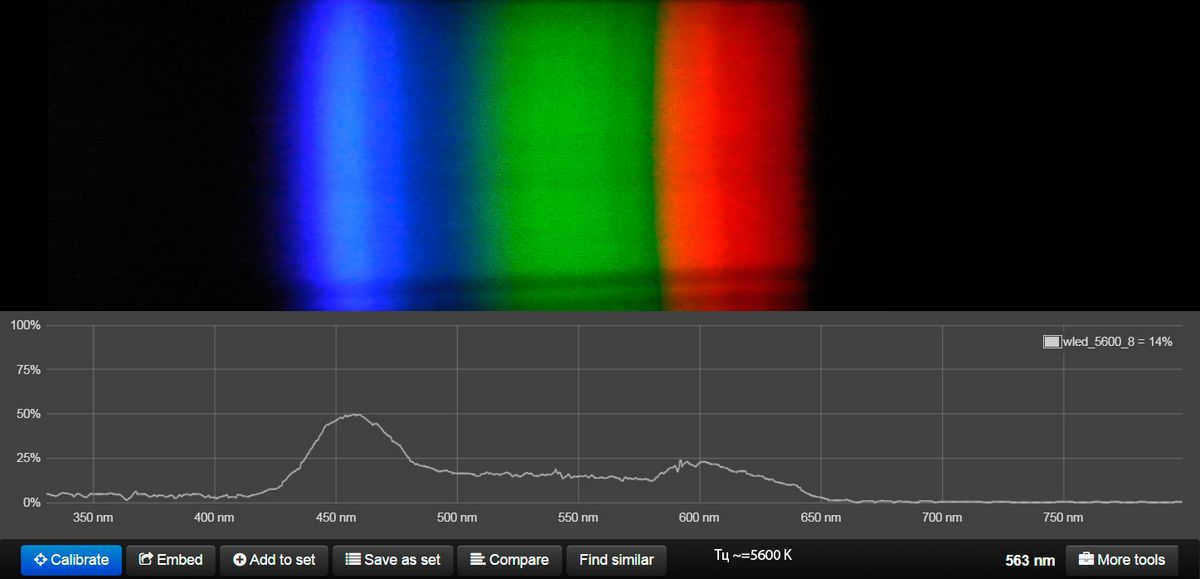
This lamp has the ability to imitate warm white, neutral white, and cool white light gradually. (hidden content) However, it does not align well with the natural solar cycle.
Here is the spectral data for the ML-19 Dual White E27 Ball 9W lamp, with its color temperature determined by the manufacturer.
Part 2 – measuring the spectrum using only your hands and blue duct tape
I have previously mentioned this topic on Hubre, but I believe it is important to give a brief explanation.
So, let’s say you and I have become fascinated with pictures of rainbows and have decided to start measuring the spectrum ourselves. However, the cheapest spectrometer in the Russian Federation costs over 70 t.r. (at the time of writing), so we will take a different approach.
Fortunately, there are some wonderful individuals on the http://publiclab.org/ website who have shared a lot of interesting open science projects. However, we are specifically interested in homemade spectrometers.
By the way, there are multiple options of spectrometers available, and you have the choice to either purchase a pre-made set for assembly (which I recommend, especially for those like me who are not very skilled) or assemble one yourself using improvised materials.
I am confident that if you have reached this point, you already have a strong desire to acquire a spectrometer, and you are eagerly awaiting a package from a country that is not particularly friendly towards us.
So, let’s discuss what we will need and what we will do:
1. First, you will need a preferably thick A4 drawing paper (such as Watman) or thick matte photo paper. In general, the denser the paper, the better. However, if you are determined, you can also try using regular paper, although it may not be as sturdy. Alternatively, if you are skilled in drafting, you can even make the spectrometer using shoe cardboard or textolite. But since I am not very skilled, I will explain my method using thick paper.
2. The printer and assembly scheme provides the option to print out the scheme and instructions on a sheet of paper. Afterward, you can happily proceed to cut out the necessary holes. It is recommended to use a sharp scalpel or blade and exercise caution while cutting.
To create the necessary components, we utilize a DVD (although a CD can also be used, the diffraction grating quality is inferior). By cutting the DVD in half with scissors, we obtain one half. Carefully dividing it into two layers, we are left with a transparent layer of photoresist. This layer needs to be cut into a square shape, matching the dimensions of the diffraction grating under our paper template. For more detailed information, please refer to this source.
3. Additionally, we bring together all the components by using a basic adhesive pencil, and secure the diffraction grating with duct tape (although double-sided tape would be preferable). Take a look at the final result – a delicate and transparent structure. If the paper used initially is thick, we can strengthen it with duct tape until we achieve a solid blue or black Malevich square. In the end, our spectroscope should effectively block out any light, with the exception of the light passing through the narrow opening.
4. It is recommended to use an old, unnecessary phone (although any phone can be used). Attach our device to the phone’s case by gluing it, making sure to position it in a way that allows the spectrum to properly fall into the camera. Alternatively, you can attach it to a webcam if you prefer. While it is possible to not glue it and instead apply it each time, this can be inconvenient and may cause the spectrum to frequently shift. The photo shows a homemade spectrometer (glued), as well as two kits – the first one is the same but from a kit, while the second one is similar in principle but made of plastic (with better quality).

We need to capture the spectrum of a compact fluorescent lamp, or alternatively, the last part of a regular fluorescent lamp. Once we have taken all the spectra we need, we must process them using a graphic editor. Typically, I crop and center them if needed. Since the calibration method (which I will explain later) involves comparing them to a standard, it is important that all spectrum bands in the pictures are in the same position as much as possible. Otherwise, the computer may interpret the shifted image as light with a different wavelength.
6. After signing up on the portal, we proceed to capture and upload the spectra. Our first step is to upload the CLL spectrum picture, which is crucial for calibration. Once the picture is successfully uploaded and the spectrum is visible, we may need to perform certain manipulations.
6.1 In case the image is not horizontally aligned, and the violet-blue zone is not on the left while the red-orange zone is not on the right, we can use additional tools such as rotation and reflection to adjust the spectrum accordingly. After that, I suggest clicking on the “re extract from foto” button (located in the tools) so that the computer can generate a curve that matches the spectrum image, although the wavelength information may still be missing.
6.2 There are two ways to correct the spectrum graph: automatically selecting the brightest spectrum from the more tools menu or manually selecting a sample row and clicking on the most accurate part of the spectrum in the image.
6.3 To calibrate the graph, press the calibrate button and then click on the top of the middle blue spectrum (as shown in the instructions). Next, select the green maximum. Now we have a calibrated standard which can be applied to other drawings loaded and processed according to step 6.1, resulting in a wavelength scale for those images as well.
6.4 To apply calibration to any image, click on calibrate -> use existing calibration and select your standard. However, please note that if you have a spectrometer attached to the camera, there may be a significant error and you may need to recalibrate.
6.5 All the subsequent data can be obtained in various formats and plotted, for example, in Excel. Although it may not be the most accurate method, it is still better than having no data at all. With enough skill, it is possible to obtain images that are consistent with reality.
Part 3 – A Brief Overview of the Impact of Light on Humans
Now, let’s briefly summarize the impact of light on humans.
Light has a significant effect on our circadian rhythms.
Specifically, light in the blue spectrum can suppress the production of melatonin, which is the hormone responsible for promoting relaxation and rest. The less melatonin we have, the more stress we may experience. This can be beneficial during the day when we need to stay alert and energized. However, in the late evening and before bedtime, exposure to blue light can disrupt our biorhythms and make it more difficult to fall asleep, leading to less efficient sleep. It’s important to note that the intensity of the light source (measured in light flux), the duration of exposure, and age (children being more sensitive and elderly people being less sensitive) all play a role in these effects.
The image depicts one of the averaged correlations between the level of melatonin suppression and the wavelength of radiation. There are other versions of this function available in related sources. (Source: Journal of Lighting Engineering, No. 3, 2012)
In summary, warm colors (such as incandescent bulbs and LED bulbs without phosphor) are more effective in creating a calming atmosphere.
For a more energizing effect, it is recommended to use daylight bulbs.
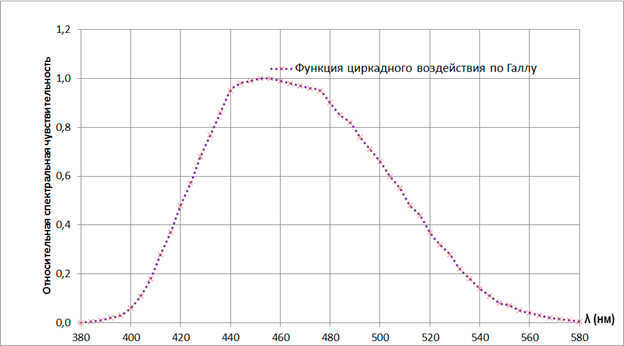
Aside from its circadian effects, light also has detrimental effects on the eyes. Excessive exposure to blue light can cause damage to the eyes, especially in children (an article discussing this can be found in the same edition of lighting technology). Therefore, it is not advisable to stare at LED or fluorescent lamps that emit cold white light for extended periods of time.
So, that’s pretty much it. The article ended up being quite lengthy, and I don’t think I’ll be able to produce something like this anytime soon. Therefore, I would like to express my gratitude to all those who took the time to read it. Please remember to take good care of your health.
All types of artificial lighting, including incandescent, fluorescent, and LED, emit light that fluctuates in intensity over time. The extent of this fluctuation, or pulsation, varies depending on the frequency at which it occurs. Our eyes may or may not be able to perceive this pulsation, but regardless, it is important to recognize that it can be harmful. To understand this, simply observe a light fixture with low-quality fluorescent lamps for a minute, and you will notice the effects (although it is rumored that if you stare at a fluorescent lamp for too long, it may start staring back at you).
There are various methods for reducing pulsation, typically involving the use of high-quality electronic power supply and control devices, such as electronic ballasts for fluorescent lamps or drivers for LEDs.
However, since it is difficult to accurately measure pulsation without specialized equipment, we will focus on the spectral characteristics of light instead. Those who are interested in measuring pulsation can find more information here.
The Sun is the primary source of light and heat that is essential for all life on Earth. However, in addition to emitting photons of light, it also releases powerful ionizing radiation composed of helium nuclei and protons. What is the reason behind this phenomenon?
Factors contributing to solar radiation
Solar radiation is generated during daylight hours when chromospheric flares occur – massive explosions that take place in the Sun’s atmosphere. As a result of these explosions, some of the Sun’s matter is expelled into outer space, forming cosmic rays that mainly consist of protons with small amounts of helium nuclei. These charged particles reach the surface of the Earth approximately 15-20 minutes after the solar flare becomes visible.
When the air intercepts the initial cosmic radiation, it triggers a chain reaction of nuclear particles that diminishes as it moves closer to the ground. This process creates a fresh batch of particles called pions, which eventually break down and transform into muons. These muons have the ability to penetrate the lower levels of the atmosphere and descend to the surface, where they can burrow as deep as 1500 meters. The muons play a crucial role in the development of secondary cosmic radiation and the natural radiation that has an impact on human beings.
 The Range of Solar Radiation
The Range of Solar Radiation
The spectrum of solar radiation encompasses both the short-wave and long-wave regions:
- gamma rays;
- X-rays;
- ultraviolet radiation;
- visible light;
- infrared radiation.
As a result of this phenomenon, the solar radiation does not cause a significant rise in radioactive radiation levels on the surface of our planet. The combined impact of the Sun’s rays and cosmic rays on the overall annual radiation dose amounts to a mere 0.3 mSv/year. However, it is important to note that this figure represents an average value, and in reality, the intensity of radiation reaching the Earth varies depending on the geographical location.
Where is the solar ionizing radiation stronger?
The poles experience the highest levels of solar ionizing radiation, while the equator experiences the lowest levels. This phenomenon occurs because the Earth’s magnetic field redirects charged particles from space towards the poles. Furthermore, radiation levels increase with altitude. At an elevation of 10 kilometers above sea level, the intensity of radiation can increase by 20-25 times. Individuals residing in high mountain regions are particularly susceptible to higher doses of solar radiation due to the thinner atmosphere, which is more penetrable by gamma rays and elementary particles emitted by the sun.
Important. The impact of radiation levels up to 0.3 mSv/h is not significant. However, at a dose of 1.2 µSv/h, it is advisable to evacuate the area. In case of an emergency, it is recommended to stay on its territory for no longer than six months. If the readings are twice as high, the stay in the area should be limited to three months.
If the altitude above sea level is 0.3 mSv/year, the annual dose of cosmic radiation exposure increases by 0.03 mSv/year with every hundred meters of elevation. By doing some calculations, we can conclude that a week’s vacation in the mountains at an altitude of 2000 meters will result in an exposure of 1mSv/year, which is nearly half of the total annual limit (2.4 mSv/year).
It has been discovered that individuals who reside in mountainous regions are exposed to an elevated level of radiation annually, which exceeds the standard limit by several times. This would lead one to believe that these individuals are more susceptible to developing leukemia and cancer compared to those who live in flat areas. However, this assumption is incorrect. On the contrary, regions with high altitudes actually have a lower mortality rate when it comes to these diseases, and some inhabitants even exhibit longevity. This serves as evidence that extended periods spent in areas with high radiation activity do not adversely affect the human body.
Solar flares – a significant peril of radiation
Solar flares pose a substantial risk to humans and all living organisms on Earth due to the intense density of solar radiation, which can surpass the typical levels of cosmic radiation by a thousandfold. Notably, the distinguished Soviet scientist A. L. Chizhevsky established a correlation between sunspot activity and the occurrence of epidemics such as typhus (1883-1917) and cholera (1823-1923) in Russia. Based on his findings, he accurately predicted in 1930 the onset of a widespread cholera pandemic between 1960 and 1962, which originally erupted in Indonesia in 1961 and swiftly spread to other nations across Asia, Africa, and Europe.
Infrequent occurrences of significant solar flares happen approximately every 4 years. During these periods, there is an increase in both the quantity and magnitude of sunspots, resulting in the formation of potent coronal rays in the solar corona. These rays are composed of protons and a limited number of alpha particles. In 1956, astronomers observed the most intense solar flare, which caused a 4-fold increase in cosmic radiation density on the Earth’s surface. Another notable outcome of this heightened solar activity was the appearance of the aurora borealis, which was documented in Moscow and the Moscow region in 2000.
What precautions can be taken?
While the increased radiation levels in the mountains should not deter individuals from visiting, it is important to consider safety measures. One such measure is to bring along a portable radiometer, which can help monitor the radiation levels and limit exposure in hazardous areas. It is recommended to avoid staying in areas where the meter readings show ionizing radiation levels of 7 µSv/h for more than a month.
The sun plays a vital role on Earth, providing essential factors such as light and heat. However, it is important to understand solar radiation, the spectrum of sunlight, and its impact on us and the global climate.
Understanding Solar Radiation
When we hear the term “radiation,” negative connotations often come to mind. However, solar radiation is actually a positive and essential phenomenon – it refers to sunlight! Every living organism on our planet relies on solar radiation for its existence. Not only does it provide warmth to our planet, but it also serves as a vital energy source for plant life.
Solar radiation encompasses all the light and energy that emanates from the sun, and it manifests in various forms. Within the electromagnetic spectrum, the sun emits different types of light waves. These waves can be likened to the ebb and flow of ocean waves, constantly moving and traveling from one location to another. The sun’s light spectrum can exhibit varying intensities, with ultraviolet, visible, and infrared radiation being the main categories.
Light is an energy in motion
The solar radiation spectrum can be compared to the keys on a piano. On one end, there are low notes, while on the other end, there are high notes. The electromagnetic spectrum follows the same pattern. On one end, there are low frequencies, and on the other end, there are high frequencies. Low-frequency waves have longer wavelengths over a given period of time. Examples of these waves are radar, television, and radio waves. On the other hand, high-frequency emissions are waves with shorter wavelengths and higher energy. Examples of these waves include gamma rays, x-rays, and ultraviolet rays.
One can conceptualize the situation in the following manner: low-frequency waves can be compared to ascending a hill with a gradual incline, while high-frequency waves are akin to rapidly scaling a steep, nearly vertical hill. Simultaneously, the height of each hill remains constant. The amount of energy carried by an electromagnetic wave is determined by its frequency. Electromagnetic waves with longer wavelengths and therefore lower frequencies possess considerably less energy than those with shorter wavelengths and higher frequencies.
The portion of the spectrum that is visible to us and other animals is positioned near the center. It’s important to note that just because we can’t perceive other types of waves, it doesn’t mean they don’t exist. Interestingly, insects have the ability to see ultraviolet light, which is beyond our visible light range. As a result, flowers appear differently to them compared to how they appear to us. This distinction plays a crucial role in helping insects determine which plants to approach and which ones to avoid.
The origin of all energy
We often take sunlight for granted, but it doesn’t have to be that way because, in reality, all energy on Earth relies on that enormous, radiant star at the heart of our solar system. Additionally, we should also express gratitude towards our atmosphere, as it absorbs a portion of the radiation before it reaches us. This delicate equilibrium is crucial: an excessive amount of sunlight would cause the Earth to become excessively hot, while too little would result in freezing temperatures.
As solar radiation passes through the atmosphere, it emits energy in various forms near the Earth’s surface. Let’s now examine the diverse methods through which this energy can be transmitted:
- Conduction refers to the transfer of energy through direct contact. For example, if you accidentally touch a hot frying pan with your bare hand and get burned, that’s an example of conduction. The heat from the pan is transferred to your hand through direct contact. Similarly, when you step on the cold bathroom tiles in the morning and feel the coldness, that’s also conduction in action as the heat from your feet is transferred to the tiles through direct contact.
- Dissipation, on the other hand, is the transfer of energy through currents in a liquid or gas. The process is the same regardless of whether it’s a liquid or a gas. When the fluid is heated, its molecules become excited, spread out, and become less dense, causing them to rise. As the fluid cools down, the molecules fall back down, creating a continuous current pathway, similar to the cells in a honeycomb structure.
- Radiation occurs when energy is transmitted in the form of electromagnetic waves. Imagine the pleasant sensation of sitting by a campfire and feeling the comforting warmth radiate towards you – this is an example of radiation. Radio waves, light waves, and heat waves are able to travel from one location to another without the need for any material medium.

The primary spectrums of solar radiation
The sun emits various types of radiation, ranging from X-rays to radio waves. Solar energy consists of both light and heat. Its composition is as follows:
- Approximately 6-7% is ultraviolet light,
- About 42% is visible light,
- 51% is near infrared.
We receive solar energy at a rate of 1 kilowatt per square meter at sea level for several hours each day. Approximately half of the radiation falls within the visible short-wave section of the electromagnetic spectrum. The remaining half is in the near-infrared portion, with a small portion in the ultraviolet section of the spectrum.
Ultraviolet radiation
Ultraviolet radiation in the solar spectrum, with an intensity greater than others, ranges from 300-400 nm. When this radiation is not absorbed by the atmosphere, it can lead to tanning or sunburn for individuals exposed to sunlight for extended periods. The effects of ultraviolet radiation from the sun on human health can be both beneficial and detrimental. It serves as a significant source of vitamin D.
Perception of Light
The perception of light plays a crucial role in understanding the solar spectrum. Researchers are particularly interested in accurately measuring the intensity and distribution of visible and near-infrared light in the electromagnetic spectrum. This information is vital for studying the impact of solar activity on our planet.
The visible range, which spans from 380 to 780 nm, is easily detectable by the human eye. This is because the majority of solar radiation energy is concentrated within this range, making it essential for maintaining the Earth’s atmospheric equilibrium. Additionally, sunlight is a critical component in the process of photosynthesis, which allows plants and other autotrophic organisms to convert light energy into chemical energy for survival.
The range of infrared spectrum, which extends from 700 nm to 1,000,000 nm (1mm), encompasses a significant portion of the electromagnetic radiation that reaches our planet. Within the solar spectrum, infrared radiation is divided into three distinct types, categorized by their wavelengths:
Summary
Various organisms, including humans, possess sensitivities that span from around 400 to 700 nm. For humans specifically, the range of colors that can be perceived is between 450 and 650 nm. Furthermore, the composition of the solar spectrum alters not only during sunrise and sunset but also in accordance with the intensity of sunlight reaching the Earth.
Solar radiation is now being viewed as a potential alternative energy source due to the fact that every fortnight, the Sun provides our planet with a sufficient amount of energy to sustain all its inhabitants for an entire year.

Light that is emitted by the Sun and travels through space is known as sunlight. Sunlight is made up of various colors, including visible light, ultraviolet rays, and infrared radiation.
Living organisms on Earth rely on sunlight as a source of energy, and it also has a significant impact on climate and weather patterns.
Helpful resources:
Understanding the nature of light
A closer look at the color red and its different shades
Explore our collection of informative articles
Sunlight consists of different types of electromagnetic radiation, which are:
- visible light,
- ultraviolet radiation,
- infrared radiation
- and radio waves.
Visible light is the most prominent component of sunlight and encompasses all the colors of the rainbow, ranging from red to violet.
Ultraviolet radiation is found beyond the visible spectrum and can pose a threat to human health if not shielded by specialized filters.
Infrared radiation falls on the lower end of the electromagnetic spectrum and is utilized in the fields of medicine and industry for temperature measurement and other purposes.
Radio waves are also present in sunlight, although they are imperceptible to humans and are employed in radio and satellite communications.

Characteristics of sunlight
Sunlight possesses several unique properties that are crucial for sustaining life on Earth.
- Energy. Sunlight contains an immense amount of energy that can be harnessed to generate electricity and power various devices.
- Photosynthesis. It serves as the primary source of energy for photosynthesis, a process that takes place in plants and other organisms.
- Temperature. Sunlight can reach extremely high temperatures, particularly near the equator.
- Humidity. It can also be utilized to measure air humidity.
- Safety. When used in accordance with safety regulations, sunlight poses no danger to humans or animals.
- Color. Sunlight encompasses a broad spectrum of colors, including ultraviolet rays, which can be harmful to the skin and eyes.
- The environment is greatly influenced by sunlight, which is responsible for photosynthesis and the balance of oxygen and carbon dioxide in the atmosphere.
Spectrum
The spectrum of sunlight determines its wavelength, which can range from 0.1 to 1 mm. Sunlight can be divided into three main spectral ranges: visible light (400-700 nm), ultraviolet light (200-400 nm), and infrared light (700-2000 nm). Each of these ranges has its own unique wavelength and is utilized in various fields such as photography, medicine, science, and industry.
Applications of solar energy
Solar energy is harnessed for a multitude of purposes, ranging from electricity generation and building heating to water and food production, as well as medical and cosmetic applications. However, it is crucial to be cautious of excessive exposure to ultraviolet (UV) radiation, which can result in skin cancer and other illnesses. Therefore, it is essential to use protective gear when working outdoors or in environments with high levels of UV radiation.
The utilization of solar energy spans a wide range of fields and brings numerous benefits to both humans and the environment. Some of the most prevalent applications of solar energy include:
- Solar panels – harness the power of sunlight to produce electricity. This method stands as one of the most effective ways to convert solar energy into usable electrical energy.
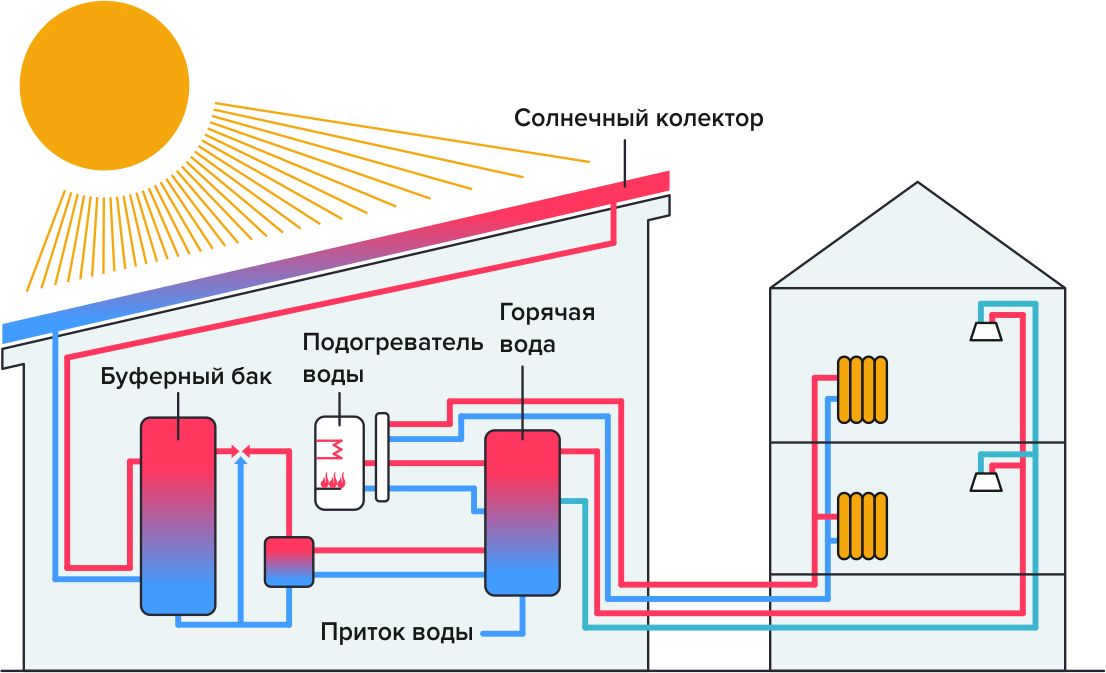
- Heating – Some structures and residences utilize solar collectors to heat water or air, resulting in reduced heating expenses and decreased greenhouse gas emissions.
- Cooling – Solar cooling systems harness the energy of the sun to lower indoor temperatures, which is particularly beneficial for air conditioning during hot weather.
- Hydropower – Solar ponds and hydroelectric plants utilize the power of falling water to generate electricity.
- Hydroponics – Utilizing sunlight to cultivate plants without soil can be advantageous for agriculture and food production.
- Cosmetology – Sunlight can be employed to address specific skin conditions and enhance skin health.
- Medicine – It can be used to treat certain ailments such as psoriasis and certain types of cancer.
- Cosmetic treatments – Ultraviolet rays are utilized for tanning and skin treatment.
- Utilizing sunlight, certain products such as wine, cheese, and yogurt can be produced through food production.
- The production of energy is achieved through the utilization of photovoltaic cells in the field of photovoltaics.
Risks Associated with Sunlight
Sunlight is a potent source of energy that can be utilized for treating numerous ailments. However, excessive exposure to sunlight can pose health risks, particularly for individuals with sensitive skin, young children, and the elderly.
Below are some of the potential dangers that can arise from prolonged sun exposure:
- Sunburn. Overexposure to ultraviolet (UV) rays can result in sunburn, which presents as reddened, tender, and painful skin. Extended periods of sun exposure can cause severe skin damage and even increase the risk of developing skin cancer.
- Risk of Skin Cancer. Prolonged exposure to UV rays heightens the likelihood of developing skin cancer, a condition that can affect both adults and children.
- Risk of Eye Diseases. The sun’s rays can harm the eyes and contribute to the development of cataracts, glaucoma, and other related conditions.
- Exposure to the sun for a long time can increase the risk of developing heart disease in individuals who already have a pre-existing condition.
- Not using proper UV protection while being exposed to the sun for an extended period of time can result in dehydration, which can lead to various issues such as fatigue, headaches, weakened immune system, and more.
To prevent these risks, it is important to use sunscreen products correctly, wear sunglasses and hats, and avoid spending too much time in the sun during its peak hours (10 a.m. to 4 p.m.).


When the word radiation is mentioned, negative thoughts usually come to mind. However, solar radiation is actually a beneficial phenomenon – it is sunlight! All living creatures on our planet rely on it. Sunlight is vital for our survival, as it not only warms the Earth but also provides nourishment for plants.
Solar radiation refers to the entirety of the sun’s light and energy and encompasses a variety of forms. Within the electromagnetic spectrum, the sun emits various types of light waves, similar to the undulating waves observed in the ocean. These waves propagate in a vertical and horizontal motion, traversing from one location to another. The sun’s light spectrum exhibits a range of intensities, with ultraviolet, visible, and infrared radiation being the primary distinctions within this spectrum.
Light is an energy that is in constant motion
The solar radiation spectrum can be compared to the keys of a piano. On one end, there are low notes, and on the other end, there are high notes. The same concept can be applied to the electromagnetic spectrum. One end consists of low frequencies, while the other end consists of high frequencies. Low-frequency waves have longer wavelengths for a given period of time. Examples of low-frequency waves include radar, television, and radio waves. On the other hand, high-frequency waves have shorter wavelengths and are characterized by high energy. Gamma rays, x-rays, and ultraviolet rays are examples of high-frequency emissions.
This is the reason why x-rays and ultraviolet radiation can be hazardous. They possess so much energy that if they penetrate the human body, they can harm cells and result in issues such as cancer and alterations in DNA. On the other hand, radio and infrared waves, which carry significantly less energy, do not have any impact on us. This is advantageous because you definitely do not want to expose yourself to risk simply by turning on the stereo.
The visible light that we and other animals can perceive with our eyes is situated nearly in the middle of the electromagnetic spectrum. Although we cannot perceive other types of waves, it does not imply that they do not exist. In fact, insects can see ultraviolet light, but they are unable to perceive our visible light. Flowers appear very dissimilar to them compared to how they appear to us, and this helps them distinguish which plants to approach and which ones to avoid.
Preventing rickets with shortwave rays
Dr. Danil Sergeyevich Simanovsky, pediatrician:
– Sunlight plays a crucial role in everyone’s health, especially children. Unfortunately, in St. Petersburg, we don’t get much sun, especially during winter and early spring. However, our body’s daily rhythms are closely tied to sunlight. That’s why it’s so difficult for children to wake up for school and kindergarten on dark mornings in St. Petersburg. During the dark months, children are more prone to developing various nervous system disorders and parents often notice a decline in their children’s immunity.
Ultraviolet rays of shorter length, known as the anti-rachitic spectrum, play a crucial role in children’s health. These rays stimulate the production of vitamin D in the skin, which is essential for proper growth and development, especially for the bones. However, it’s important to note that vitamin D alone doesn’t directly promote bone growth. Instead, it acts as a facilitator, ensuring that calcium is transported to the appropriate locations in the body, at the right time.
An excess of vitamin D in children poses a greater risk than a deficiency. As a result, parents, especially those with children under one year old, should consult their pediatrician to discuss the appropriate vitamin D regimen based on seasonal and light level changes. The typical preventive dose, which does not require constant monitoring, is 400-500 IU (international units) per day. If high doses of vitamin D are being administered alongside artificial ultraviolet irradiation and consumption of vitamin D-enriched foods, it is crucial to confirm the suspected vitamin D deficiency and closely monitor the treatment. A specialized blood test is necessary to measure vitamin D levels.
Excessive vitamin D intake is more dangerous for children than a deficiency
When spring arrives and the sun starts to shine, the child’s body begins to produce its own vitamin D. In addition to this, the child also gets vitamin D from food and medicines. However, this can sometimes lead to an excess of vitamin D in the body, causing a decrease in blood calcium levels. This excess can result in symptoms such as muscle twitching, muscle pain (especially at night), and even seizure-like movements. This condition is known as spasmophilia. It is common for parents and doctors to increase the dose of vitamin D in response to these symptoms, but this can actually make the situation worse. Therefore, it is important to calculate the amount of vitamin D your child is receiving from sources such as infant formula, fermented milk products, baby porridge, and vitamin-enriched mashed potatoes before going for a preventive checkup with the pediatrician in the spring.
The Origin of All Energy
We often take the sun for granted, but it’s important to remember that all energy on Earth relies on this massive, luminous star at the heart of our solar system. Additionally, we should express our gratitude to the atmosphere, as it acts as a buffer, absorbing some of the sun’s radiation before it reaches us. This delicate balance is crucial: too much sunlight and the Earth becomes scorching hot, too little and it plunges into freezing temperatures.
As solar radiation passes through the atmosphere and reaches the Earth’s surface, it releases energy in a variety of forms. Let’s examine the different methods through which it can be transferred:
- Conduction, also known as heat conduction, occurs when thermal energy is transferred through direct contact. For example, if you accidentally touch a hot frying pan without wearing an oven mitt, the heat from the pan is transferred to your hand through conduction. Similarly, when your bare feet come into contact with the cold bathroom tiles in the morning, heat is transferred from your feet to the tiles through conduction.
- Dissipation, on the other hand, involves the transfer of energy through currents in a liquid or gas. When a liquid is heated, its molecules become excited and dispersed, causing it to become less dense and rise. As the liquid cools down, the molecules fall back down, creating a cellular current pathway.
- Radiation occurs when energy is transmitted in the form of electromagnetic waves. Consider the pleasant sensation of sitting by a campfire and feeling the comforting warmth radiating towards you – this is an example of radiation. Various types of waves, such as radio waves, light waves, and heat waves, can travel from one location to another without the need for any medium.

What is the composition of the Sun?
If one were to dissect a star and analyze its elemental makeup, it would become evident that the Sun is primarily composed of 74% hydrogen and 24% helium. Additionally, the Sun contains 1% oxygen, with the remaining 1% consisting of various chemical elements from the periodic table, including chromium, calcium, neon, carbon, magnesium, sulfur, silicon, nickel, and iron. Astronomers classify any element heavier than helium as a metal.
Further reading: Step-by-step instructions and recipes for colon cleansing enemas using oil and herbs
The interior of the Sun undergoes a process known as the proton-proton cycle
How were these components of the Sun formed? The Big Bang gave rise to the creation of hydrogen and helium. During the early stages of the Universe’s formation, hydrogen, the first element, originated from elementary particles. The conditions in the Universe at that time, characterized by high temperature and pressure, resembled those found in the core of a star. Subsequently, hydrogen underwent fusion reactions to produce helium, facilitated by the ongoing high temperatures in the universe. The current proportions of hydrogen and helium present in the Universe were established after the occurrence of the Big Bang and have remained unchanged.
The creation of the most massive elements, such as gold and uranium, took place during the explosive demise of stars that were significantly larger than our Sun. In an astonishingly brief period of time after the birth of a black hole, these elements collided with tremendous force, resulting in the formation of the heaviest elements known to us. Following the cataclysmic explosion, these elements were dispersed across the vast expanse of the universe, ultimately contributing to the formation of new stars.
Throughout its existence, our Sun has diligently accumulated a diverse assortment of elements, ranging from those forged in the crucible of the Big Bang and remnants of dying stars to particles originating from the explosive detonations of freshly formed stars.
The primary spectra of solar radiation
The Sun emits a wide range of radiation, spanning from X-rays to radio waves. Solar radiation consists of both light and heat. The composition of solar radiation can be broken down as follows:
- Approximately 6-7% of ultraviolet light
- About 42% of visible light
- 51% of near infrared
At sea level, we receive solar energy with an intensity of 1 kilowatt per square meter for numerous hours each day. Roughly half of this radiation falls within the visible shortwave portion of the electromagnetic spectrum, while the other half is in the near infrared range, with a small fraction in the ultraviolet region.
A brief description
| What? | How much? |
| The distance between the Earth and the Sun | Average – approximately 149.6 million kilometers (maximum – 152 million kilometers, minimum – 147 million kilometers). |
| The temperature of the Sun’s surface | Around 5500°C-6000°C |
| Sun’s diameter | About 1.392 million kilometers |
| Solar mass | 1.988 × 10^30 kilograms |
| Sun’s radius | 696 thousand kilometers |
| Sun’s density | The average density is approximately 1.408 grams per cubic centimeter. |
| Age of the Sun | About 5 billion years |
Perception of Light
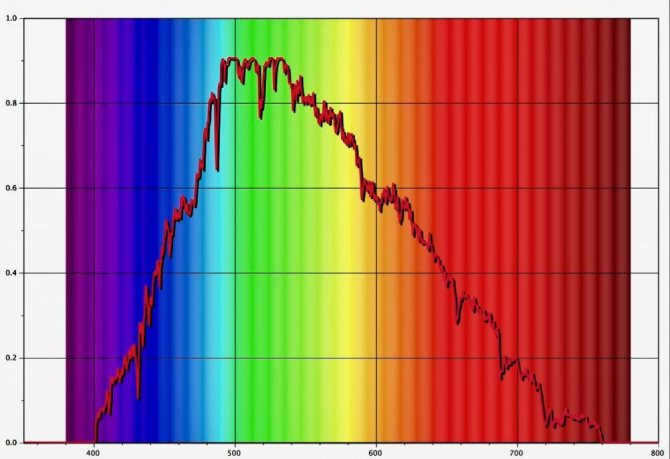
The perception of light in the solar spectrum is characterized by its average intensity. The study of solar-terrestrial effects requires a quantitative understanding of the flux and variations of its spectral distribution in the visible and near-infrared ranges of the electromagnetic spectrum. The range from 380 to 780 nm is perceivable to the human eye.
This specific range is significant because it contains the majority of solar radiation energy, which plays a crucial role in determining the thermal equilibrium of the Earth’s atmosphere. Sunlight is essential for the process of photosynthesis, enabling plants and other autotrophic organisms to convert light energy into chemical energy that can be utilized as fuel.
Radiative transfer zone
The radiative transfer zone is situated immediately following the core and covers a distance of 0.7 solar radius. In this region, thermal convection is absent, however, the solar material is incredibly hot and densely packed, allowing for the efficient transfer of intense heat through thermal radiation from the core to the outer layers. Predominantly composed of hydrogen and helium ions, photons are emitted and travel a short distance before being absorbed by other ions.
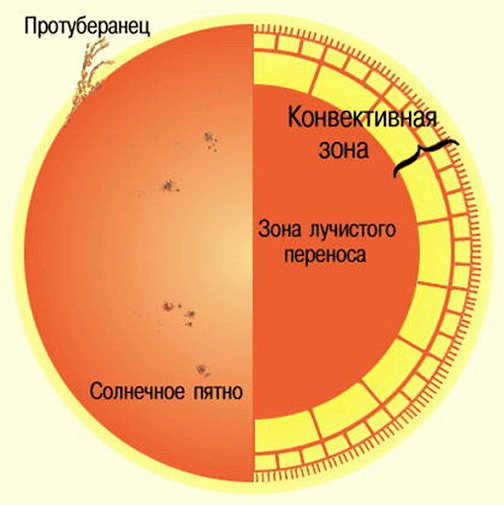
The temperature in this region is lower, ranging from approximately 7 million degrees Celsius towards the center to 2 million degrees Celsius towards the outer boundary of the convective zone. Likewise, the density decreases by a factor of one hundred, going from 20 g/cm³ near the center to 0.2 g/cm³ at the upper limit.