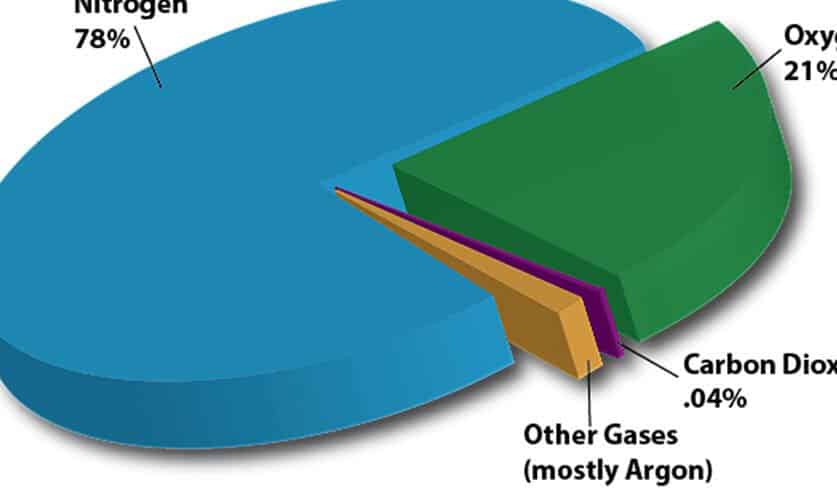
The Earth’s atmosphere is made up of a combination of various gases. The majority of the atmosphere is composed of nitrogen, accounting for 77 percent, while oxygen makes up an additional 21 percent. The remaining 2 percent consists of a mixture of trace gases such as argon, carbon dioxide, helium, neon, krypton, xenon, nitrous oxide, carbon monoxide, and others. Additionally, the atmosphere contains varying concentrations of water vapor. Oxygen is particularly important for human life, as we rely on this gas to survive.
In some cases, premature babies with underdeveloped lungs may be placed in oxygen tanks where they breathe a mixture with a higher oxygen content. Instead of the typical 21 percent concentration, these tanks can reach levels of 30 to 40 percent oxygen. In instances of severe respiratory distress, the child may breathe pure oxygen to prevent damage to brain cells.
Did you know: Having too much oxygen in the gas we breathe can be just as harmful as not having enough.
Excessive Oxygen and Oxidation Hazards
Having too much oxygen can be just as perilous as having too little. Excessive oxygen levels in the gas mixture and high concentrations of oxygen in the bloodstream can lead to the destruction of the eye tissues in infants, resulting in vision loss. This highlights the dual nature of oxygen – it is essential for our survival, but it can also act as a poison to living organisms. When oxygen in the atmosphere reacts with other elements like hydrogen and carbon, it triggers a process called oxidation. Oxidation breaks down the organic molecules that form the foundation of life. At normal temperatures, oxygen reacts slowly with other elements, and the resulting heat is so negligible that we don’t perceive it.
Temperature and oxidation
The relationship between temperature and oxidation is a crucial aspect to consider in various industries. Temperature plays a significant role in the rate of oxidation reactions. As temperature increases, the rate of oxidation generally accelerates. This is due to the fact that higher temperatures provide more energy for the reactant molecules, allowing them to collide more frequently and with greater force. These collisions result in increased reaction rates and oxidation processes. Therefore, controlling temperature is essential in industries where oxidation reactions are undesirable, such as in the production of certain chemicals or in food storage. By maintaining lower temperatures, the rate of oxidation can be slowed down, reducing the risk of spoilage or degradation. On the other hand, in some industries where oxidation is desired, such as in the production of certain metals or during combustion processes, higher temperatures are necessary to promote oxidation. Overall, understanding and controlling the relationship between temperature and oxidation is crucial in various industrial processes.
However, when the temperature rises, oxidation reactions speed up. To ignite a match, you strike it against the abrasive strip on the box. This creates friction, which in turn heats up the match head. As a result, the oxidation reaction occurs rapidly and the match quickly bursts into flames. During this reaction, you can see the light and feel the heat that is released.
In our bodies, oxidation is a less dramatic process. Red blood cells absorb oxygen from the air in the lungs and transport it throughout the body. Under strictly controlled conditions, oxygen in living cells oxidizes the food we consume at a much slower rate and with less intensity than a burning match. This oxidation process breaks down the food, releasing energy, and producing water and carbon dioxide. The carbon dioxide is then carried by the blood to the lungs, where it is expelled into the atmosphere through exhaled air.
Did you know? The yellowing of book pages is caused by oxidation, a slow burning process.
Oxygen is essential for life. It is necessary for breathing and without it, we can suffocate within five minutes. In fact, cutting off the oxygen supply is an effective way to extinguish a fire, as demonstrated by using a thick blanket. The atmosphere contains an ideal level of oxygen at 21 percent. However, oxygen can also be dangerous. For instance, a spark can cause dry grass to ignite into flames. In nature, the balance of oxygen and other gases is maintained through the life cycles of plants and animals. Animals exhale carbon dioxide, which is absorbed by plants that release oxygen in return.
Oxygen levels
There is no certainty, however, that the oxygen levels will remain constant for centuries as they are at present. The quantity of carbon dioxide being released into the atmosphere is on the rise. This emission originates from fossil liquid fuels, such as gasoline and fuel oil, which generate carbon dioxide nitrogen upon combustion. Simultaneously, the forests, which serve as the Earth’s green lungs, are being rapidly depleted. In just one minute, numerous hectares of forest are being cleared. This combination results in a decline in the oxygen concentration in the atmosphere, and scientists are striving to find ways to minimize the harm caused to it.
Engrossing video on the Earth’s atmosphere
If you happen to come across any mistakes, simply select the erroneous text and hit Ctrl+Enter.

Forces and particles
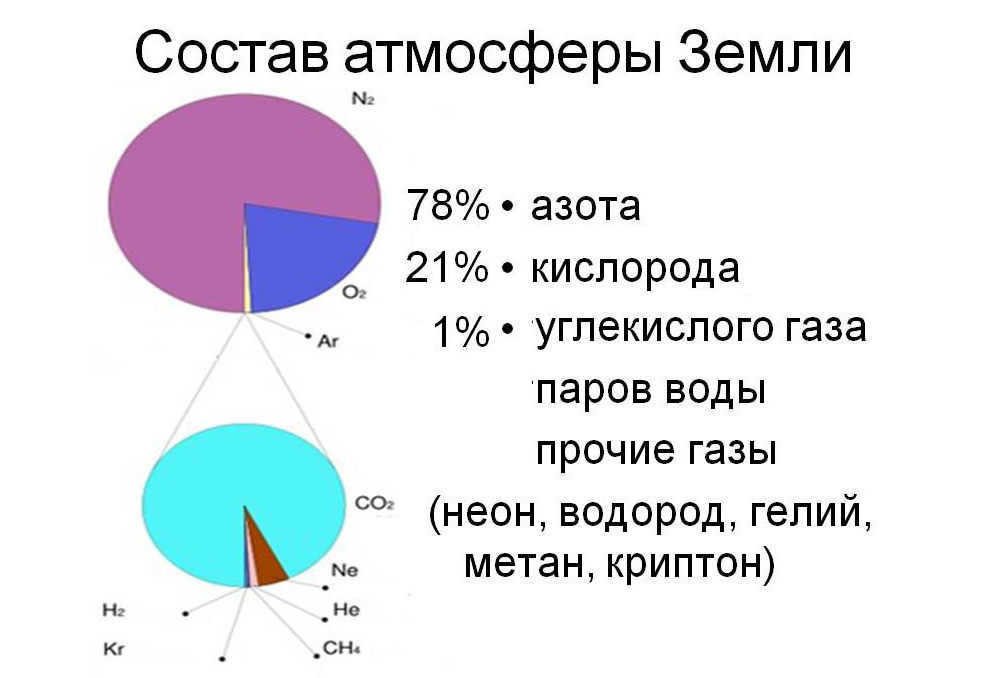
When contemplating the Earth’s atmosphere, our initial thought is that it consists mostly of oxygen. However, in reality, our planet’s atmosphere is a blend of various gases. If you were to randomly seize an air molecule, chances are it would be a nitrogen molecule.
The makeup of the Earth’s atmosphere

This gas makes up 77 percent of the Earth’s atmosphere. Oxygen makes up 21 percent. The rest of the volume consists of small amounts of other gases and water vapor. Some of these other gases have specific uses; for example, carbon dioxide is used in the production of carbonated beverages, neon is used in electric lighting, helium is used to fill balloons, methane and krypton are also present, nitrous oxide is known as “laughing gas” and is used as an anesthetic in surgery, hydrogen and ozone are also found, as well as xenon and more.
Molecules of gases
The air contains a mixture of molecules of gases, both common and very rare, which are evenly distributed throughout (although the majority of ozone is found in a thin layer of the atmosphere approximately 24 kilometers above the Earth’s surface). These molecules move around us at speeds ranging from 1,100 to 4,800 kilometers per hour. The distances they travel are so small that they cannot be seen with the naked eye. While you are reading this, it is possible that a molecule of krypton has entered your nasal passage.
Just picture a billion equally divided parts of a centimeter. One of those parts happens to be roughly the size of an air molecule’s diameter. Molecules are actually bundles of atoms, or rather, bunches of atoms. The size of a molecule increases as more atoms are connected together. For instance, a water molecule comprises two hydrogen atoms and one oxygen atom. However, there are large molecules, like the DNA molecules found in animal and plant cells, which consist of millions upon millions of atoms.
Air molecules come in different sizes, with most of them being incredibly small. To put it into perspective, an oxygen molecule consists of two oxygen atoms, while a nitrogen molecule is composed of two nitrogen atoms. These molecules are so minuscule that they are measured in units of hundred millionths and billionths of a centimeter. The concept of dividing an inch into such tiny pieces is mind-boggling. However, we can gain some understanding of the size of air molecules by comparing them to table salt crystals, as proposed by scientists Gerald Feinberg and Robert Shapiro in their book Life Beyond Earth.
Comparison between the size of air molecules and salt crystals
Imagine having a pile of salt on the table in front of you. Select the tiniest crystal from the pile. Now, picture yourself as Alice in Wonderland. As you start to shrink rapidly, the salt crystal becomes increasingly larger. Eventually, it grows to the size of a baby’s crib. Then, it expands to the dimensions of a house. Finally, it reaches towards the sky.
However, you are still not sufficiently diminutive to perceive the sensation of an air particle. You are still unable to comprehend the relative size of a particle when compared to a granule of salt. Slowly, the apex of the salt crystal vanishes from sight. From your perspective, it now appears as tall as one hundred skyscrapers aligned vertically. And all of a sudden, you spot a tiny piece of lint and attempt to capture it. Thus, what you hold in the palm of your hand is an average air particle. By juxtaposing this with a granule of salt towering one hundred skyscrapers high, you can truly grasp the nature of a molecule.
A fascinating video about the Earth’s atmosphere
If you happen to come across any mistakes, kindly select the specific text and hit Ctrl+Enter.
It may come as a surprise to some individuals that oxygen is not the most abundant gas in Earth’s atmosphere. When considering the relative quantities of gases in Earth’s atmosphere, nitrogen actually surpasses oxygen by over threefold.
Arranged in descending order of percentage, the chemical makeup of Earth’s atmosphere includes nitrogen, oxygen, argon, CO 2, and trace gases. While water vapor also constitutes a significant portion of the atmospheric composition, its amount varies greatly depending on geographical location and season, and therefore is excluded from the overall total and treated separately.
Found at the lowest layer of the atmosphere, the troposphere holds 75 percent of the atmosphere’s mass.
Nitrogen (78.1%): The Dominant Component of Earth’s Atmosphere
The primary gas present in the earth’s atmosphere is nitrogen, with a composition of 78.1 percent. Despite being the most abundant gas in the atmosphere, nitrogen only constitutes 0.005% of the Earth’s crust by mass.
Nitrogen exhibits remarkable stability and requires a significant amount of energy to undergo any changes in its physical state.
While its overall presence in the Earth’s crust may be relatively small, nitrogen plays a crucial role in the nitrogen cycle, which involves a constant exchange of nitrogen between the atmosphere and living organisms.
What is the amount of oxygen present in the Earth’s atmosphere? Oxygen occupies the second position and constitutes approximately one-fifth of the entire atmosphere, precisely 20.9%. The Earth possesses the ideal conditions for the flourishing of life. Oxygen plays a crucial role in human life as our lungs inhale oxygen and utilize it in metabolic processes.
Despite nitrogen being an incredibly stable gas, it poses difficulties in separation and utilization within chemical processes. Oxygen readily engages in chemical reactions as it serves as a significant participant in all oxidative processes.
Therefore, even though nitrogen is abundant, we require oxygen to drive the chemical reactions that generate energy.
Argon (0.93%)
Argon, which makes up 0.93% of the Earth’s atmosphere, is a chemical element with the symbol Ar and atomic number 18. It is a noble gas that is colorless, odorless, and tasteless. Argon is the third-most abundant gas in the atmosphere, after nitrogen and oxygen. It is used in various applications, such as in incandescent light bulbs and in the production of lasers. Argon is also used as a shielding gas in welding and as an inert gas in laboratory settings. Despite its abundance, argon is relatively inert and does not readily react with other elements.

Argon is an unreactive gas that does not interact with or impact the composition of the Earth’s atmosphere. In fact, argon makes up only 0.93% of the atmosphere, which is why it does not participate in any atmospheric cycles. However, nitrogen and carbon are present in the atmosphere because they have the ability to form chemical bonds with other elements.
Argon, being an inert gas, does not bind to or affect the atmosphere. And, with a concentration of only 0.93% in the Earth’s atmosphere, it does not participate in any argon cycles. However, nitrogen and carbon are present in the atmosphere due to their capacity to form bonds with other elements.
Carbon dioxide (0.04%):
Carbon dioxide, with a concentration of 0.04%, is a significant component of the Earth’s atmosphere. This greenhouse gas plays a crucial role in regulating the planet’s temperature, as it traps heat and prevents it from escaping into space. Carbon dioxide is produced through natural processes such as respiration and volcanic activity, but human activities such as burning fossil fuels have significantly increased its levels in the atmosphere. This increase in carbon dioxide has led to the intensification of the greenhouse effect and is a major contributor to global warming. It is important for us to find ways to reduce carbon dioxide emissions and mitigate the impacts of climate change.
Carbon dioxide (CO2) is the primary gas impacted by human activity. In return, the carbon in the CO2 molecule is the key element for constructing the molecules essential for living organisms.
As illustrated by the long-term carbon cycle, carbon assumes various forms including carbon dioxide (CO2), methane (CH4), and glucose (C6H12O6).
There is currently much discussion about the greenhouse effect resulting from the substantial emissions of CO2, but in reality, its overall proportion of the total air volume is quite small, amounting to only about 0.04%.
Since 1900, the amount of carbon dioxide has primarily increased due to human activity. After extracting fossil fuels, humans proceed to burn them.
Consequently, gases like methane and carbon dioxide contaminate the atmosphere. In fact, the quantity of carbon dioxide has nearly doubled since 1900.
Trace gases present in the atmosphere of Earth
The remaining portion of the Earth’s atmosphere consists of trace gases, such as neon, helium, and methane. Methane and krypton are among the primary trace gases that constitute a small fraction of the atmosphere.
In addition to naturally occurring trace gases, human activities can also release certain trace gases into the atmosphere. One example is chlorofluorocarbons (CFCs), which contribute to ozone layer depletion at the poles.
When chlorine enters the troposphere and eventually reaches the stratosphere, it reacts with ozone (O3), leading to its depletion. Water vapor, like ozone, is a variable gas in the atmosphere.
Water vapor has been eliminated from the total percentage due to its regional variations. However, it can constitute a significant portion of the atmosphere. For instance, in hot regions, it can account for 5% of the volume, while in colder areas, it is much lower.
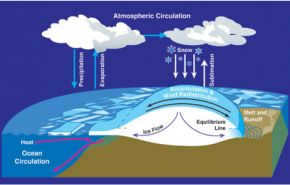
Water vapor plays a crucial role in regulating air temperature as it absorbs solar radiation. It is produced through evaporation from the Earth’s surface, including oceans, lakes, and rivers. Once in the atmosphere, water vapor undergoes condensation, often resulting in rain. It transitions from a gaseous state to a liquid state.
Water is constantly in motion as part of the hydrological cycle, and this movement is driven by solar energy.
The focus is placed on the various storage locations for water, including the atmosphere, glaciers, oceans, plants, and humans. The majority of evaporation occurs in the oceans, and it is the Coriolis effect that directs it away from the equator.
What is the distribution of gases in the atmosphere? The distribution of carbon dioxide.
In the atmosphere, the distribution of gases, including carbon dioxide, varies depending on various factors. In the northern hemisphere, we can observe higher concentrations of carbon dioxide, particularly in regions with major emission sources. These sources are mainly located in North America, Europe, and Asia. However, the gas does not remain localized to these areas due to atmospheric circulation patterns and ocean currents.
The amount of carbon dioxide in the atmosphere also experiences seasonal variations. During the spring and summer, when photosynthesis is active, plants absorb significant amounts of carbon dioxide from the atmosphere. This leads to a decrease in its concentration. However, as summer transitions into fall, photosynthesis decreases, and carbon dioxide is released back into the atmosphere. This phenomenon is influenced by Earth’s metabolism, also known as net primary productivity.

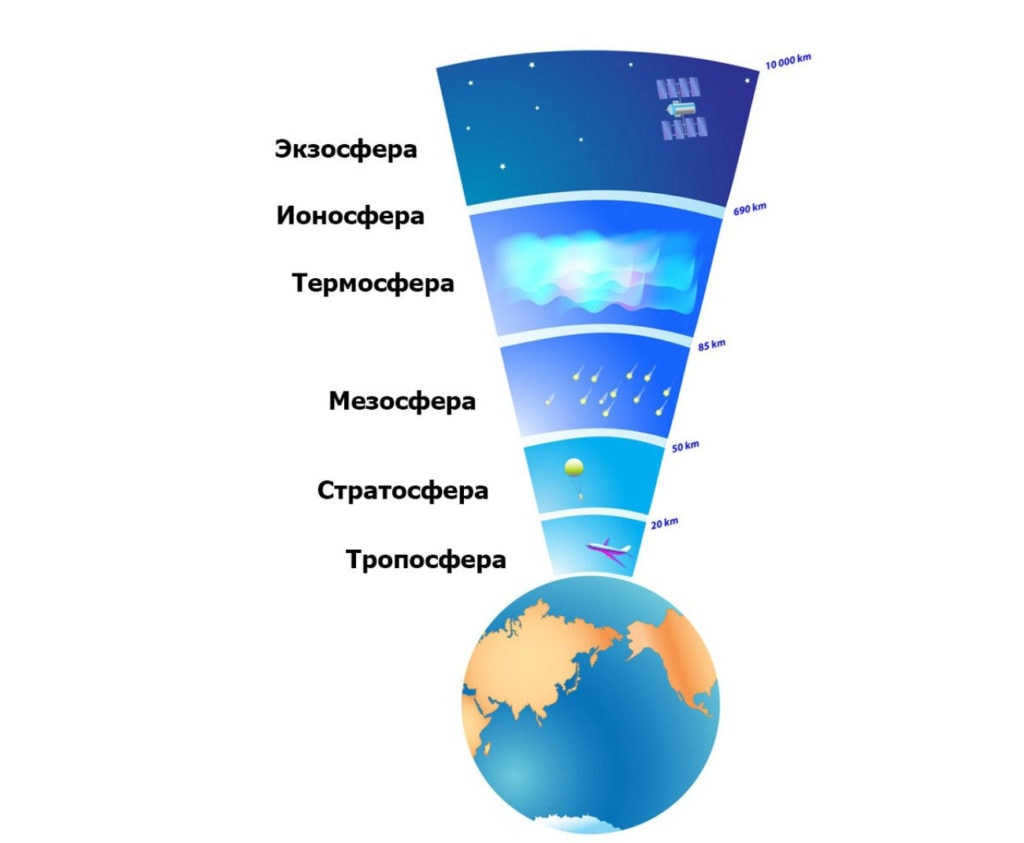
The Earth’s atmosphere consists of multiple layers of gases that encircle the planet due to the effects of gravity.
Each layer possesses a distinct gas composition and is arranged based on its density. More dense gases are drawn closer to the Earth’s surface, while less dense gases are located further away from the planet.
Due to the unique properties of each gas, the atmosphere’s layers possess their own characteristics and play specific roles in their interactions with the Earth.
The Earth’s atmosphere is composed of five layers:
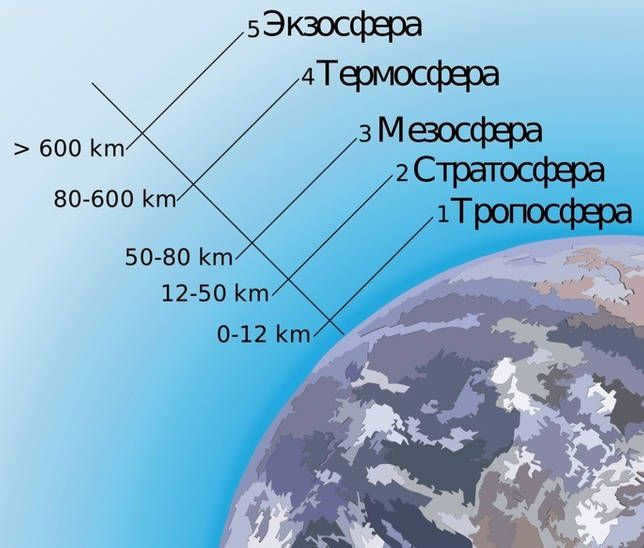
Depicting the distances of each layer from the Earth
Chemical Composition of Dry Air in Percentages
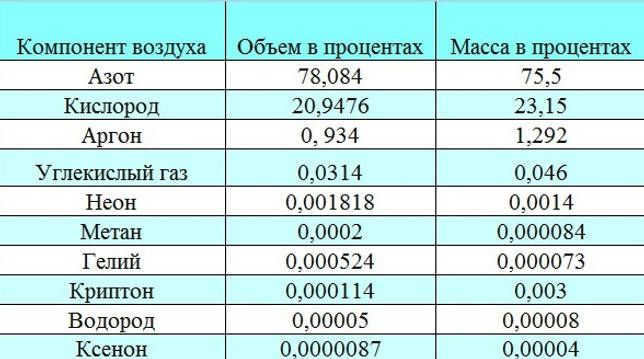
The composition of gases present is influenced by various factors:
- Weather conditions and seasonal changes,
- Geographical position
- Density and size of the population,
- Humidity and pollution levels.
Troposphere
The troposphere, being the closest and densest layer to the Earth’s surface, contains the majority of the atmosphere. It is estimated that the total mass of the atmosphere is 5×1018 kg, with 75% of this mass present in the troposphere.
The thickness of the troposphere varies from 8 km to 14 km, depending on the location on Earth. The thinnest areas, measuring around 8 km in thickness, are found at the north and south poles.
Because it serves as the innermost layer of the atmosphere, the troposphere plays a crucial role in sustaining life on Earth and is where nearly all weather phenomena take place. The term “troposphere” is derived from the Greek word “tropos” (meaning “turn” or “change”) to emphasize the dynamic nature of climate fluctuations and the behavior of this atmospheric layer.
The boundary between the troposphere and the stratosphere is known as the tropopause. The tropopause can be easily distinguished by distinct patterns of pressure distribution and temperature in each respective layer.
Structure of the troposphere
The troposphere is composed of different gases in varying proportions. Nitrogen makes up 78.08% of the volume, while oxygen accounts for 20.95%. Argon and carbon dioxide make up 0.93% and 0.04% respectively. Additionally, the troposphere also contains varying amounts of water vapor, which enters through the process of evaporation.
Temperature in the troposphere
Similar to pressure, temperature decreases as altitude increases within the troposphere. This is because the Earth’s surface absorbs the majority of the sun’s energy, heating the lower levels of the troposphere. Warmer regions tend to have higher levels of evaporation, resulting in a higher concentration of water vapor at sea level and a lower concentration at higher altitudes.
What can be observed in the troposphere?
There are several things that can be observed in the troposphere:
- Weather patterns;
- Precipitation including rain, snow, and hail;
- Gaseous elements such as nitrogen, oxygen, argon, and carbon dioxide;
- Cloud formations;
- Avian species.
The stratosphere, the Earth’s second largest layer of the atmosphere and the second closest to its surface, is estimated to contain approximately 15% of the total mass of the planet’s atmosphere.
With a thickness of 35 km from the tropopause, the stratosphere is situated between the troposphere and the mesosphere. The name “stratosphere” is derived from the Greek word “strato,” meaning “layer,” which reflects the fact that the stratosphere is divided into various thinner layers.
The formation of stratospheric layers is a result of the absence of climatic phenomena that would mix the air, leading to a clear separation between the cold, dense air below and the warm, light air above. Consequently, the stratosphere operates in direct contrast to the troposphere in terms of temperature.
Due to the fact that the stratosphere offers greater vertical stability (with minimal air movement), airplane pilots often prefer to remain within its lower regions in order to avoid encountering turbulence. It is precisely at this altitude that both airplanes and balloons are able to operate with utmost efficiency.

Several airplanes, particularly jets, ascend into the stratosphere in order to avoid air circulation.
The stratosphere additionally contains the famous ozone layer, which absorbs the majority of the sun’s UV radiation. Devoid of the ozone layer, life on Earth as we understand it would not be feasible.
Comparable to the troposphere, the stratosphere also possesses a region that marks its conclusion and indicates the commencement of the mesosphere, which is referred to as the stratopause.
Most substances present on the Earth’s surface and in the troposphere do not make their way up to the stratosphere. Instead, they typically:
- break down in the troposphere;
- can be destroyed by sunlight;
- can be brought down to the Earth’s surface through rainfall or other forms of precipitation.
Due to the temperature inversion between the troposphere and stratosphere, there is limited movement of air between the two layers. As a result, water vapor is present in the stratosphere only in minuscule amounts. Consequently, the formation of clouds is extremely rare in this region.
The stratosphere is primarily composed of ozone, which makes up the ozone layer and comprises approximately 90% of all the ozone in the atmosphere. Additionally, this region contains various elements that are transported by volcanic eruptions, including nitrogen oxides, nitric acid, halogens, and other substances.
Temperature in the stratosphere
The temperature in the stratosphere rises as the altitude increases, ranging from -51 °C at the lowest point (known as the tropopause) to -3 °C at the highest point, which is called the stratopause.
Contents of the stratosphere
Here are a few examples of what can be found in the stratosphere:
Mesosphere
The mesosphere is the uppermost layer of the Earth’s atmosphere where gases are still mixed together and not separated by their weight. This particular layer is considered to be the most challenging to study, resulting in limited confirmed information.
Located between the stratosphere and the thermosphere, the mesosphere has a thickness of approximately 35 km from the stratopause. The term “mesosphere” originates from the Greek word “mesos,” meaning “center,” as it is the third layer among the five layers of the atmosphere.
Due to its extreme altitude, meteorological instruments and aircraft are unable to reach the mesosphere. Satellites, on the other hand, can only orbit above it, making it challenging to accurately measure the properties of this layer.
Phenomena like starbursts (meteor streams) occur when celestial bodies that enter the Earth’s atmosphere burn up in the mesosphere.

When a celestial object enters the Earth’s atmosphere, it creates a meteor shower, also known as a star shower.
As a result of the intense heat, the celestial object ignites and typically breaks apart into multiple smaller pieces.
Composition of the mesosphere
The composition of the mesosphere is similar to that of the layers below, with oxygen, nitrogen, and carbon dioxide making up the majority. Unlike the stratosphere, water evaporation is less common in the mesosphere, resulting in a lower amount of ozone. However, some ozone is still transferred from the stratosphere to the mesosphere.
In addition to its atmospheric constituents, the mesosphere contains materials from meteors that vaporize upon entry. As a result, the mesosphere also contains a relatively high concentration of iron and other metals.
As altitude increases in the mesosphere, temperatures decrease. The temperature ranges from -3° C at the lowest point, known as the stratopause, to -143° C at the highest point called the mesopause, which is the coldest region of the Earth’s atmosphere.
What can be found in the mesosphere?
Here are some examples of what can be found in the mesosphere:
Thermosphere
The thermosphere is positioned above the mesosphere and below the exosphere. It spans a thickness of approximately 513 km, making it significantly thicker than all the lower layers combined.
Despite being classified as part of the Earth’s atmosphere, the thermosphere has such a low air density that a majority of the layer is often mistaken for outer space. This misconception is further supported by the lack of sufficient molecules in this region to transmit sound waves.
Within the thermosphere, the occurrence of photoionization, a process where photons interact with atoms to create ions, is driven by ultraviolet radiation. This phenomenon is responsible for the formation of the ionosphere, which is situated within the thermosphere. The ionosphere plays a crucial role in facilitating the transmission of radio waves to distant areas of the Earth.
The thermosphere is where satellites rotate around the International Space Station (ISS). Additionally, the thermosphere is where the phenomenon of the northern lights takes place.


The phenomenon known as the Northern Lights happens when solar particles collide with the density of the Earth’s atmosphere.
Discover more fascinating information about the Northern Lights.
The term “thermosphere” is derived from the Greek word thermos, which means “heat,” indicating the extremely high temperature in this layer.
The boundary dividing the thermosphere and the exosphere is referred to as the thermopause.
In contrast to the layers beneath, where gases intermingle, particles in the thermosphere have infrequent collisions, leading to a homogeneous distribution of elements. Furthermore, sunlight causes the majority of molecules in the thermosphere to decompose.
The higher regions of the thermosphere comprise of atomic oxygen, atomic nitrogen, and helium.
Temperature in the thermosphere
The temperature in the thermosphere can range anywhere from 500º C to 2000º C. This wide temperature range is due to the fact that the majority of sunlight is absorbed within this layer.
What can be found in the thermosphere?
There are various things that can be found in the thermosphere, including:
- Satellites
- The former reusable transportation spacecraft Space Shuttle
- The International Space Station (ISS)
- The breathtaking phenomenon of the northern lights
- The ionosphere
Exosphere
The exosphere, which is the largest and outermost layer of Earth’s atmosphere, spans 600 km before seamlessly transitioning into interplanetary space. It is approximately 10,000 km thick and its outermost boundary extends halfway to the Moon.
The term “exosphere” originates from the Greek word “exo,” meaning “outer,” highlighting its position as the final atmospheric layer before the vastness of the cosmic vacuum.
The exosphere contains particles that are widely spaced apart and thus cannot be categorized as gases due to their low density. It is possible for a single particle to travel hundreds of kilometers before encountering another. Additionally, these particles do not possess electrical charges, which is why they are not classified as plasma.
In the lower regions of the exosphere, one can find hydrogen, helium, carbon dioxide, and atomic oxygen, which are only weakly attracted to Earth by its gravitational field.
The temperature in the exosphere
Due to the lack of molecular interaction, the exosphere remains almost in a vacuum, resulting in a constant and cold temperature within this layer.
What can be found in the exosphere?
Some examples of the entities present in the exosphere include:
Atmospheres of celestial bodies
Within our solar system, there are 8 planets and over 160 satellites. Out of these, the following have significant atmospheres:
- Earth;
- Venus;
- Saturn;
- Mars;
- Uranus;
- Jupiter;
- Neptune;
- Titan (a satellite of Saturn);
- Pluto (a dwarf planet).
Venus’ atmosphere
Venus has an atmosphere that is approximately 96% composed of carbon dioxide, with a surface temperature reaching as high as 464 degrees Celsius. The atmosphere is also known for its sulfuric acid clouds, which move at speeds of approximately 100 meters per second.
Mars’ atmosphere
Mars, on the other hand, possesses a relatively thin atmosphere consisting of around 95% carbon dioxide, with the remaining composition consisting of nitrogen and argon. The average temperature of the surface air on Mars is a chilly -63 degrees Celsius. Interestingly, both water and carbon dioxide clouds have been observed on Mars, and the planet also experiences distinct seasons.
For more information, see Singularity and Cosmology.
General information
The term “atmosphere” is used to describe the layer of gas that surrounds our planet and other celestial bodies in the universe. It creates a protective barrier that extends several hundred kilometers above the Earth’s surface. The composition of the atmosphere is made up of a variety of gases, with oxygen being the most abundant.
- Physical diversity.
- Increased activity.
- Sensitivity to biological factors (vulnerable to adverse events).
The atmosphere plays a crucial role in sustaining life on our planet by shielding it from potential collisions with celestial objects and creating optimal conditions for the development of various life forms.
Comprising the protective layer are:
- Nitrogen – 78%.
- Oxygen – 20.9%.
- A gas mixture – 1.1% (including ozone, argon, neon, helium, methane, krypton, hydrogen, xenon, carbon dioxide, and water vapor).
The gas mixture also serves the important role of absorbing excessive solar energy. Furthermore, the composition of the atmosphere varies with altitude – at a height of 65 kilometers above the Earth’s surface, it consists of 86% nitrogen and only 19% oxygen.
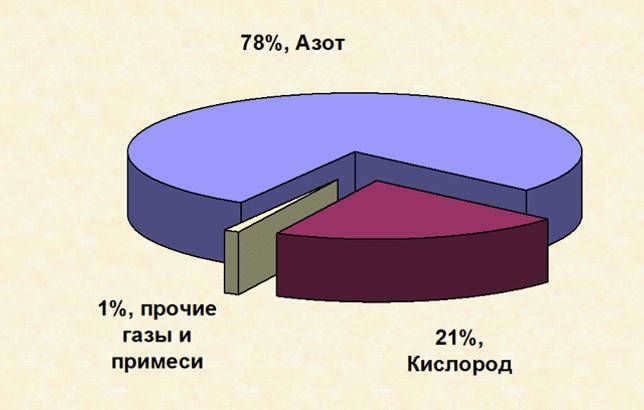
Earth’s atmosphere has a diverse composition that enables it to perform various functions and protect life on the planet. The main components of the atmosphere include:
Composition of the atmosphere
The different elements present in the Earth’s atmosphere serve important purposes. Some of these elements are:
- Carbon dioxide (CO₂) – plays a crucial role in the process of plant nutrition known as photosynthesis. It is released into the atmosphere through the respiration of all living organisms, the decay of organic matter, and combustion. Without carbon dioxide, plants would cease to exist.
- Oxygen (O₂) – provides an optimal environment for the survival of all organisms on the planet and is essential for respiration. If oxygen were to disappear, 99% of organisms on Earth would not be able to survive.
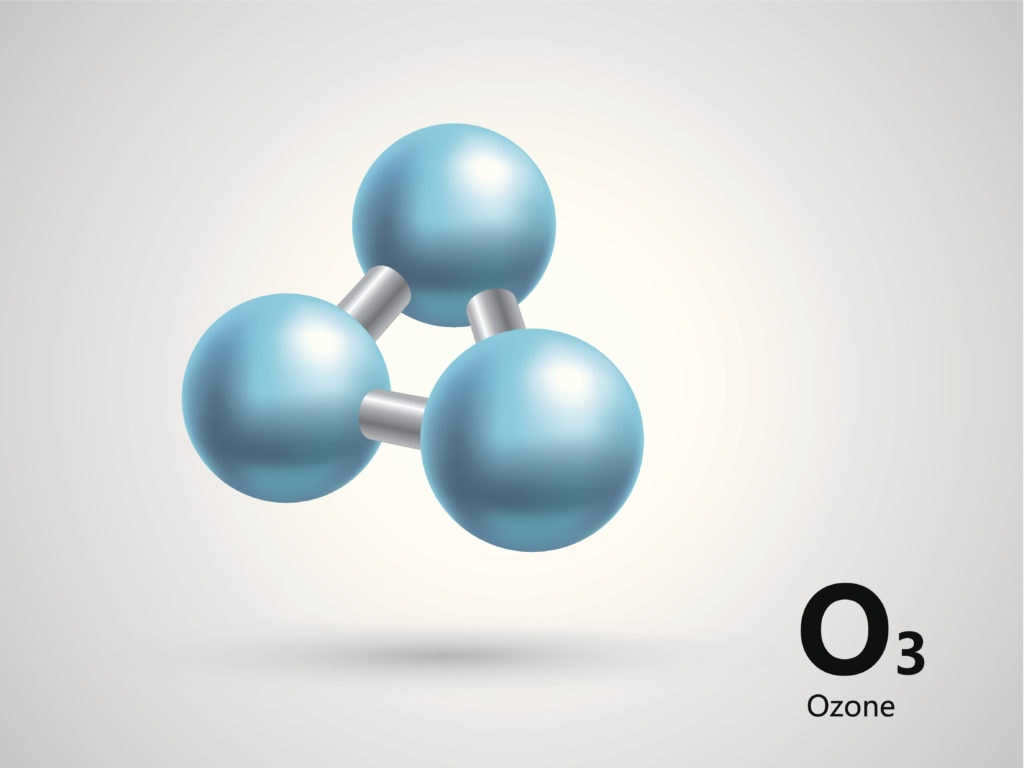
- Ozone (O3) is a naturally occurring gas that acts as a protective barrier against harmful ultraviolet radiation from the sun. However, excessive amounts of ozone can have negative effects on living organisms. The gas forms a unique layer in the Earth’s atmosphere called the ozone shield. Unfortunately, due to external conditions and human activities, this protective layer is gradually deteriorating. It is crucial to take measures to restore the ozone layer in order to preserve life on our planet.
In addition, the atmosphere also contains water vapor, which plays a role in determining the humidity of the air. The percentage of water vapor in the atmosphere can vary depending on various factors. These factors include:
The concentration of water vapor and temperature have an impact – when it is low, the concentration remains below 1%, but when it increases, it can reach levels of 3-4%.
In addition, the Earth’s atmosphere also contains solid and liquid impurities such as soot, ash, sea salt, different microorganisms, dust, and water droplets.
To gain a comprehensive understanding of the Earth’s valuable gas shell, it is imperative to have knowledge about the layered structure of the Earth’s atmosphere. These layers are characterized by varying compositions and densities of gas mixtures at different altitudes. Each layer possesses distinct chemical compositions and functions. The proper arrangement of the Earth’s atmospheric layers is as follows:
The troposphere is the layer of the Earth’s atmosphere closest to the surface. It has a height of 16-18 km in tropical areas and an average height of 9 km above the poles. Around 90% of all water vapor is found in this layer. Cloud formation occurs in the troposphere, as well as air movement, turbulence, and convection. Temperatures in the troposphere can vary, ranging from +45 degrees Celsius in the tropics to -65 degrees Celsius at the poles. With every 100-meter increase in elevation, the temperature drops by 0.6 degrees Celsius. The troposphere, with its accumulation of water vapor and air, plays a significant role in the development of cyclonic processes. Therefore, the correct answer to the question of which layer of the Earth’s atmosphere cyclones and anticyclones form in is the troposphere.
The stratosphere is situated at an altitude of 11-50 km above the Earth’s surface. In its lower region, temperature levels drop to around -55 degrees. Within the stratosphere, there exists an inversion layer, marking the transition between this layer and the mesosphere, which follows it. Temperature readings within the inversion layer can reach up to +1 degree. The lower region of the stratosphere is commonly traversed by airplanes.
The ozone layer, situated between the stratosphere and the mesosphere, plays a vital role in shielding all life on our planet from the harmful effects of ultraviolet (UV) light. Not only does it create a barrier, but it also ensures a conducive environment for living organisms, separating them from the harsh conditions of outer space where even bacteria cannot survive without specific adaptations. This protective layer is formed through the interaction of organic components and oxygen, which react to ultraviolet radiation, resulting in the formation of ozone gas. By absorbing UV light, ozone contributes to the heating of the atmosphere, thus maintaining optimal conditions for life as we know it. Therefore, the answer to the question of which gas layer safeguards the Earth from cosmic radiation and excessive solar radiation is indeed ozone.
The thermosphere is a layer of the Earth’s atmosphere situated approximately 100 kilometers above sea level, with the Karman line serving as its lower boundary. This boundary is conventionally considered the beginning of space. The thermosphere has a thickness of around 800 kilometers and experiences temperature readings of up to 1800 degrees. However, the presence of a small concentration of air helps protect the surfaces of spacecraft and rockets. Within this layer of the atmosphere, a unique phenomenon known as the northern lights occurs.
The northern lights, also referred to as aurora borealis, is a remarkable type of luminous display that can be observed in specific regions of the planet. It is caused by the interaction of various factors, including the ionization of the air and the influence of cosmic radiation and radiation upon it.
What is the outermost layer of the atmosphere? It is called the Exosphere. In this region, gases are sparsely distributed, leading to their gradual escape from the atmosphere. The Exosphere is situated approximately 700 km above the Earth’s surface. The primary constituent of this layer is hydrogen. Other substances like oxygen or nitrogen may exist in atomic form and become highly ionized due to solar radiation.
The exosphere of the Earth extends up to 100 thousand kilometers from the planet.
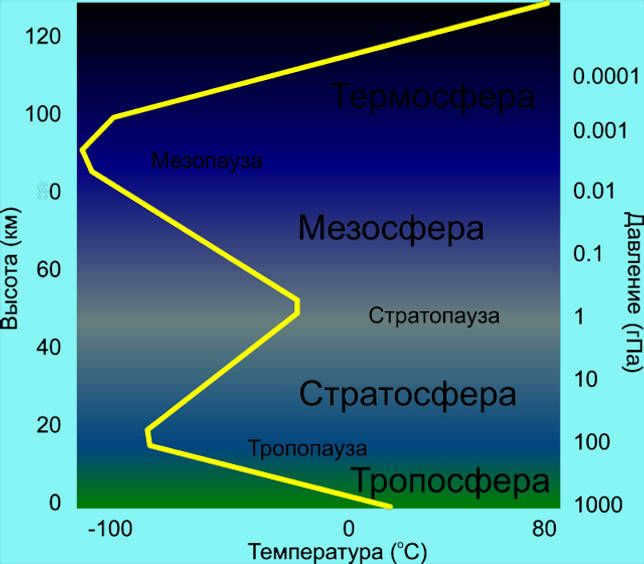

The Earth’s atmosphere consists of various gases.

Air is a combination of gases that are essential for life and the maintenance of life on Earth. What are the characteristics of air and what substances make up its composition?
Composition of Air
Air is necessary for all living organisms to breathe. It is made up of nitrogen, oxygen, argon, carbon dioxide, and various impurities. The composition of the atmosphere can vary depending on the conditions and the environment. For instance, in urban areas, the level of carbon dioxide in the air is higher compared to forested areas due to the abundance of vehicles. At high altitudes, the concentration of oxygen decreases because nitrogen molecules are lighter than oxygen molecules. As a result, the concentration of oxygen decreases more rapidly.

If we discuss the percentage composition of air, the primary constituent is nitrogen, which makes up 78% of the total air volume. Oxygen, on the other hand, accounts for 20.9% of the air molecule. Nitrogen and oxygen are the two primary elements found in air. The concentration of other substances is significantly lower, not exceeding 1%. For instance, argon occupies 0.9% of the air volume, while carbon dioxide comprises just 0.03%. Additionally, air contains impurities such as neon, krypton, methane, helium, hydrogen, and xenon.
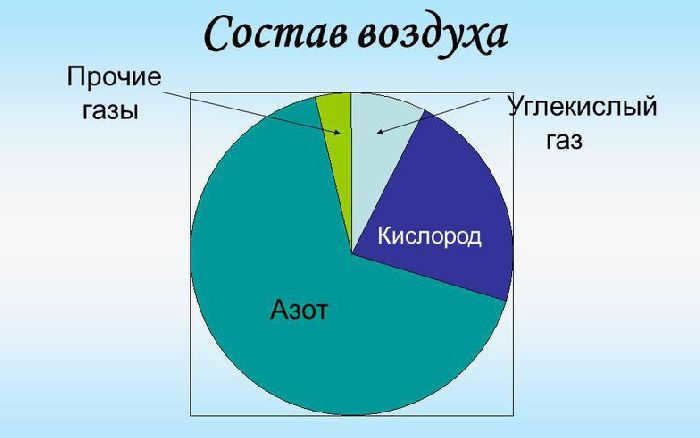
The aeroionic composition of the air is highly valued in industrial settings. The presence of negatively charged ions in the air can have a positive impact on the human body, providing an energizing effect and boosting mood.
Nitrogen
Air is predominantly composed of nitrogen. Although the name of this element translates to “lifeless,” nitrogen plays a vital role in various biological processes. It is a crucial component of proteins, nucleic acids, and vitamins, among other essential compounds.
Being an element of the second period, nitrogen does not have any excited states due to the absence of free orbitals in its atom. However, nitrogen can exhibit a valence of III or IV in its ground state through the formation of covalent bonds using the donor-acceptor mechanism involving its unshared electron pair. Nitrogen’s oxidation states can range from -3 to +5, showcasing its versatility.
Nitrogen is found in nature both as a simple substance – N2 gas, and in a bound state. The nitrogen molecule consists of nitrogen atoms that are connected by a powerful triple bond, with a bond energy of 940 kJ/mol. Under normal conditions, nitrogen can only interact with lithium. However, when molecules are activated through heating, irradiation, or catalysts, nitrogen can react with both metals and nonmetals.
Oxygen is the most prevalent element on our planet: it makes up 47.3% of the Earth’s crust by mass and 20.95% of the atmosphere by volume. In living organisms, oxygen comprises approximately 65% of their mass.
In nearly all compounds, except for those containing fluorine and peroxides, oxygen maintains a constant valence of II and an oxidation state of -2. The oxygen atom does not have any excited states because there are no unoccupied orbitals at the second outer level. As a pure element, oxygen exists in two different forms: oxygen gas (O2) and ozone gas (O3). The most significant compound containing oxygen is water. Approximately 71% of the Earth’s surface is covered by water, and life as we know it would be impossible without it.
Ozone is naturally formed when oxygen in the air undergoes lightning discharges, and it can also be produced in the laboratory by passing an electrical discharge through oxygen.
Ozone is a more powerful oxidizing agent compared to oxygen. Specifically, it has the ability to oxidize gold and platinum.
In the field of industry, oxygen is typically acquired by the process of air liquefaction, followed by the separation of nitrogen through vaporization. The separation is based on the difference in boiling points, with liquid oxygen having a boiling point of -183 degrees and liquid nitrogen having a boiling point of -196 degrees.