
Throughout history, scientists from various regions of the world have developed numerous hypotheses to explain specific processes, phenomena, and occurrences. Some of these hypotheses were quickly proven or disproven through practical experimentation. Others remained purely theoretical for many years due to the limitations of technology at the time. In this article, we will explore a recent study conducted by researchers from Goethe University Frankfurt in Germany. Their aim was to investigate the true nature of “light pressure” while also validating a theory proposed nearly 90 years ago. We will delve into the details of this theory, the experimental techniques employed, and the new insights gained regarding photons. The scientists’ findings will be presented here. Let us proceed.
Historical context
Electromagnetic radiation pressure, also known as light pressure, refers to the mechanical force applied to a surface when momentum is exchanged between an object and an electromagnetic field.
The individual credited with discovering this concept is Johannes Kepler (1571-1630). While observing a comet in 1619, he observed that its tail always pointed away from the Sun.
It wasn’t until 1862, over two hundred years later, that James Maxwell (1831-1879) proposed that light, as a form of electromagnetic radiation, possesses momentum and thus exerts pressure on any surface it interacts with. Experimental confirmation of this theory didn’t occur until 1900, when Peter Lebedev conducted experiments to validate Maxwell’s ideas.
Conducting practical experiments to investigate the phenomenon of light pressure proves to be an arduous task. The main challenge stems from the minuscule magnitudes of the forces generated by this pressure. Nonetheless, on a cosmic scale, these seemingly negligible forces can accumulate over time and exert a significant impact on objects. To illustrate, had the calculations preceding the launch of the Viking program spacecraft failed to account for light pressure, the vehicle would have remained in orbit around Mars, positioned approximately 15,000 km away from the planet.

Johannes Kepler, Peter Lebedev and Arnold Sommerfeld.
When we combine all the information, we arrive at the following conclusion: photons, which are particles of light, collide with the atoms of an object and transfer a portion of their momentum to it, causing the object to accelerate.
And now, after 90 years, our contemporaries have had the opportunity to witness this enigmatic phenomenon for the very first time in history.
The authors of the study highlight that the electric field vector of the electromagnetic wave is perpendicular to the axis of light propagation. This field is responsible for driving photoionization. It can be inferred that its direction serves as the axis of symmetry for the angular distributions of photoelectrons and photoions.
Photoionization* – The process of ionizing a molecule/atom directly through the absorption of photons with energy equal to or greater than the ionization energy.
photoeffect – The process of interaction between electromagnetic radiation and matter, where the energy of photons is transferred to the electrons of the matter.
Photoelectron refers to the electrons that are displaced from a substance when it is exposed to electromagnetic radiation.
Photoion refers to a cation (positively charged ion) that is produced by photoionization.
However, when the photon energies Eγ and photon pulses kγ are high, this symmetry is broken. As a result, the momentum distributions of the reaction fragments become asymmetric in relation to the direction of light propagation.
In his investigations, Sommerfeld discovered that the average forward momentum of electrons (⟨k e x⟩ > kγ) means that the mean momentum of the photoion must be in the opposite direction in order to conserve momentum.
It should be pointed out that the non-dipole effects resulting from the non-zero momentum of the photons also play a significant role in single-photon ionization. Moreover, the higher multipole components of the interaction between light and matter not only alter the angular distribution of photoelectrons, but also create additional ionization pathways that would not be possible with dipoles alone.
In this investigation, two variations of single-photon ionization experiments were conducted:
- Low-energy (300-1775 eV) experiments were carried out at PETRA III (DESY/German Electron Synchrotron) using circularly polarized light;
- High-energy (12-40 keV) experiments were performed at ID31 (European Synchrotron Radiation Facility) using linearly polarized light.
The supersonic gas jet of He (low-energy experiment) or N2 (high-energy experiment) intersected the photon beam perpendicularly. An electric field directed the ions towards a detector that can measure both time and position, with the position being indicated by the delay line*.
Delay line* – A device that can postpone electrical and electromagnetic signals for a specific period of time.
The initial pulses were generated based on the flight time of the ions and the location of the contact point. In the experiments involving N2, ionization of the K-shell (the outermost electron shell) was followed by an Auger* decay.
Auger* effect – The release of an electron from an atom’s shell through a non-radiative transition when the excitation is removed.
When this situation occurs, two ions with a single charge are generated and coincide with the Auger electron. Using these three momentum vectors, the momentum of ion N was calculated2 + at the moment immediately after the emission of the photoelectron.
To accurately measure the ion pulses on an absolute scale, it is crucial to determine the exact position of the zero-pulse ions on our detector. For high-energy data, this reference point is determined by the ions generated by Compton scattering..
Compton scattering* – refers to the incoherent scattering of photons on free electrons, where the photons before and after scattering do not interfere with each other.
In this scenario, the momentum of the photon is transferred to the electron, resulting in the ion having a momentum distribution centered around the initial point.
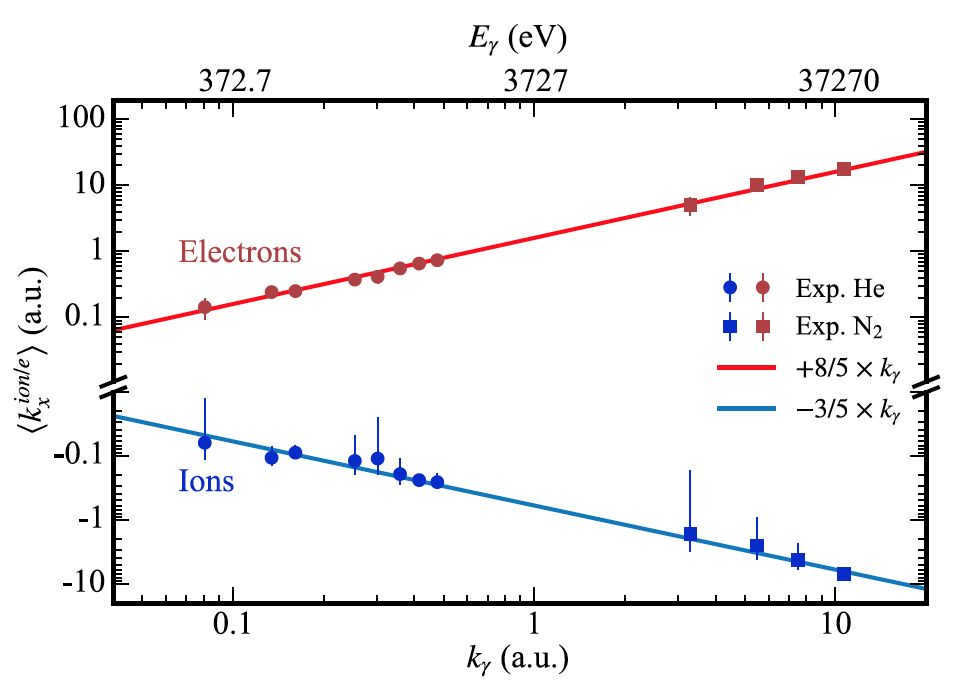
Image #1
The data presented in the diagram represents the findings of the research. The graph displays the average momentum value of the ion in the direction of light propagation ⟨k ion x⟩. The blue color indicates this value as a function of photon energy (upper scale) or photon momentum (lower scale). The dots on the graph represent single ionization of He at low photon energies, while the squares represent ionization of the K-shell N2 at high photon energies.
When the momentum is negative, it suggests back emission, which means the emission is in the opposite direction from the photon propagation. The red color on the graph represents the mean value of the photoelectron momentum ⟨k e x⟩, which is obtained by considering momentum conservation based on the measured momentum of the ion.
The predicted data is represented by the red and blue lines, which are calculated using the following formulas:
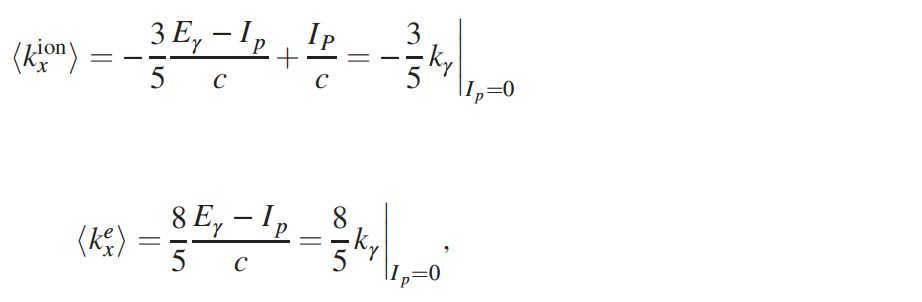
The ionization potential, denoted as Ip, and the speed of light, denoted as c, are involved in this measurement.
Based on the information provided, it can be concluded that this serves as a tangible demonstration of the theory regarding the emission of ions in photoionization that occurs in the opposite direction of the incident light.
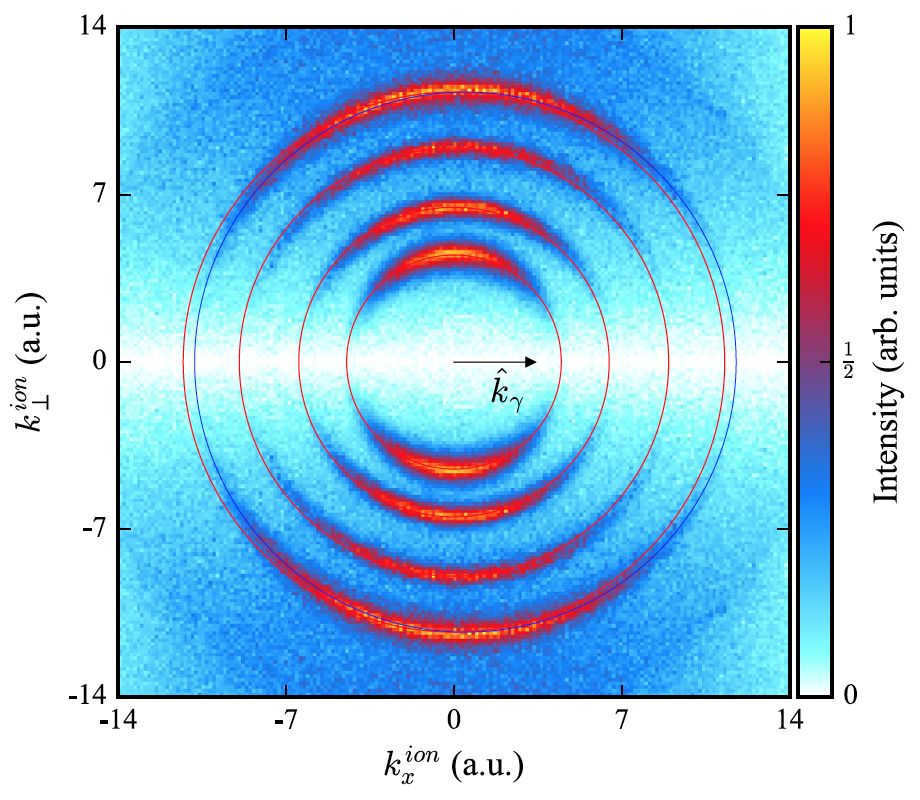
Image #2
The provided image displays the distribution of photoion momentum for the process of photoionization of helium. In this experiment, photons with circular polarization at energy levels of 300, 600, 1125, and 1775 eV were utilized. The horizontal axis represents the momentum component parallel to kγ, while the vertical axis represents the momentum perpendicular to the photon axis. The red concentric rings, centered at the same position as the initial momentum space point, are clearly visible. The radius of these rings is determined by the corresponding photoelectron impulses ke = √ 2(Eγ – Ip).
Ionization events on these rings do not accumulate, but instead they move forward in the direction of photon propagation. This phenomenon is most noticeable in the outer ring, which corresponds to a photon energy of 1775 eV. In this particular case, the blue rings are moved forward by the momentum of the 1775 eV photon.
As a result, the momentum distributions of the ion, as measured, clearly demonstrate that the majority of the photon momentum is absorbed by the ion, which is a direct consequence of momentum conservation.
During each individual ionization event, the photon momentum is transferred to the center of mass of the system, which is almost coincident with the ion. The corresponding momentum distribution of the electron shows a circle of the same radius, but it is not shifted forward.
Aside from the ring’s forward shift in ion momentum space, the momentum distribution on the ring also varies with photon energy. As the energy Eγ increases, this distribution deviates more towards the hemisphere opposite the forward direction.
Momentum conservation dictates that the final momentum of the measured ion equals the momentum of the photon minus the momentum of the photoelectron. As a result, the distribution of ions on the shifted sphere in momentum space and the angular distribution of photoelectrons in the laboratory reference frame are exact mirror images of each other (image #3).
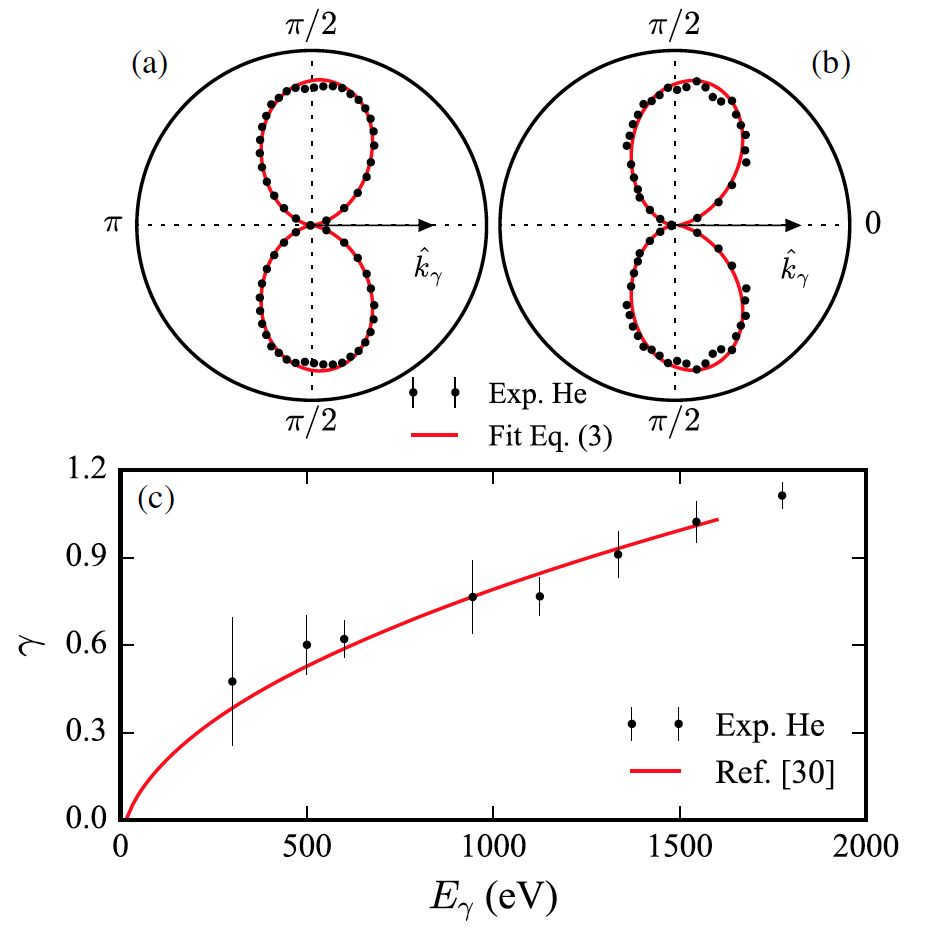
Image #3
The shape of the particles is approximately dipole-like because the initial state is He(1s), and as a result, the primary component of the angular momentum in the final state is a dipole. Moreover, this dipole shape is deflected forward.
According to the authors of the study, there are numerous explanations in the literature regarding the transfer of photon momentum, but many of them are inaccurate. The most common explanation is that the absorbed photon transfers its own momentum to the ejected electron. Based on this explanation, it is believed that this “shock” is responsible for the forward shift in the angular distribution of electrons, as depicted in the image above.
To better grasp the intricacies, scientists propose keeping in mind the precise mechanism of how a photon transfers momentum when interacting with an electromagnetic field. For the sake of simplicity, the photoionization of the 1s-electron of the hydrogen atom has been selected as an illustrative example.
Going beyond the electric dipole approximation, the ionizing plane’s electromagnetic wave with wave vector kγ = kγ = Eγ/c (photon momentum) “impresses” the local phase factor e ikγ-r onto the element of the transition matrix.
By inputting the RH coordinate for the center of mass of the atom and the r′ coordinate for the electron 1s relative to RH, we can restate the absolute coordinate of electron 1s in the laboratory reference frame as r = RH + r′. Thus, we can express the corresponding phase as follows: e ikγ-r = e ikγ-RH e ikγ-r′ .
This stage, which is symbolized by a domain, alters a component of the transition matrix: the initial factor from the equation above enters the transition matrix element ⟨π e ikγ-RH π 0⟩ between the transition states of the atomic center of mass, which are described by plane waves (2π) -3/2 e iπ-RH with momentum π. This magnitude creates the principle of momentum conservation π = π0+kγ. Hence, when an atom absorbs a photon, it imparts an impulse kγ to its center of mass.
The second phase factor e ikγ-r′ from the equation is accountable for the multipole modifications beyond the electric dipole approximation.
Based on the results, it is evident that for initial states s, the backward momentum of the ion scales -(3/5)kγ, providing confirmation to Sommerfeld’s theory.
If you want a more in-depth understanding of the intricacies of the research, I suggest taking a look at the scientists’ report.
Conclusion
Coming up with formulas and developing theories is no simple task, but finding evidence to support or refute these theories can be even more challenging.
In this study, researchers were able to validate a theory that was formulated in the 1930s. The authors of the paper not only measured the ion’s pulse, but also determined its origin. The source of this momentum is known as the “recoil” of the ejected electron.
According to scientists, if the photon has a low energy level, its momentum can be ignored in theoretical modeling. However, when the photon has high energy, this neglect leads to significant inaccuracies. Experimental data has helped determine the threshold at which the momentum of the photon cannot be ignored.
In the future, scientists plan to continue this research, as groundbreaking discoveries provide a more comprehensive understanding of the processes that occur during the distribution of energy between two or more photons.
Thank you for your attention, stay curious, and have a productive week, everyone.
A touch of publicity
Thank you for choosing to stay connected with us. Are you enjoying our articles? Would you be interested in exploring more captivating content? Show your support by making a purchase or recommending us to your acquaintances. Discover our exceptional cloud VPS for developers starting from just $4.99 – an exclusive alternative to basic servers, thoughtfully designed for your needs. Unveil the complete truth about our VPS (KVM) E5-2697 v3 (6 Cores), equipped with 10GB DDR4, 480GB SSD, and a 1Gbps connection, all starting from $19. Or, learn about the proper server division techniques with our RAID1 and RAID10 options, available with up to 24 cores and a maximum of 40GB DDR4.
Is the Dell R730xd 2x cheaper at Equinix Tier IV data center in Amsterdam? Exclusively with us, get the Dell R730xd with 2 x Intel TetraDeca-Core Xeon 2x E5-2697v3 2.6GHz 14C 64GB DDR4 4x960GB SSD 1Gbps 100 TV starting at $199 in the Netherlands! Also, don’t miss out on the Dell R420 – 2x E5-2430 2.2Ghz 6C 128GB DDR3 2x960GB SSD 1Gbps 100TB starting at $99! Discover how to build an enterprise-class infrastructure using Dell R730xd E5-2650 v4 servers that typically cost 9000 euros for just a fraction of the price!
Science fiction authors often depict spaceships powered by solar wind, which may seem far-fetched. However, in reality, light does exert pressure on objects it encounters.


Understanding Light Pressure
If you direct a stream of air towards a piece of paper, you can easily observe that the gas exerts pressure on the surface. Similarly, when a stream of light is directed onto paper or any other surface, it also exerts pressure, although significantly less.
This phenomenon can be explained using two theories.
In the field of modern physics, it is believed that light particles, also known as photons, possess dual properties:
- The properties of an electromagnetic wave (wave theory of light).
- The properties of a material particle (corpuscular theory).
According to the theory of corpuscles, photons bombard a surface like flying balls, transferring their momentum to the object being irradiated and generating pressure.
Who was the first to measure the force of pressure exerted by light?
The discovery of light pressure is attributed to the Russian physicist Pyotr Nikolayevich Lebedev. However, this phenomenon was actually predicted by Johannes Kepler in the 17th century based on his astronomical observations. Maxwell, the founder of classical electrodynamics, proposed that a stream of light could exert pressure and explained it using the wave theory of light.


Lebedev’s most significant contribution to the field of physics was his experimental confirmation of the existence of light pressure and his ability to accurately measure its magnitude. Prior to this breakthrough, the scientific community was divided into two camps:
- One group believed in the existence of light pressure, but lacked the technical means to prove it at the time;
- The other group, including the esteemed William Thomson, believed that light did not exert any pressure.
Both sides presented compelling arguments, and the only way to settle the debate was through a successful experiment that would be universally recognized.
A calculation formula
In 1873, Maxwell derived a theoretical formula for the pressure of light in his book “Treatise on Electricity and Magnetism.”

In the formula above, the following variables are defined:
- E represents the amount of energy in the light flux per unit time, measured with respect to the surface area;
- R stands for the reflection coefficient;
- c denotes the speed of light in a vacuum.
The goal of the researchers was to measure the quantities involved in this equation and verify or refute Maxwell’s findings.
Efforts to carry out experiments in order to demonstrate the validity of either side, came to naught, despite the simplicity of the concept. It was proposed to direct a beam of light onto a lightweight wing suspended in a vacuum, and calculate the desired value based on the angle at which the wing was deflected.
When surfaces are exposed to light, there were other forces that disrupted the outcomes of experiments. For instance, a portion of the radiation was absorbed, resulting in the heating of the object being illuminated. This heating was typically uneven, leading to non-uniform heating of the surrounding gas. Convection phenomena that arose as a result had an additional impact that surpassed the power of the light flux. To address this, it was necessary to place the object being irradiated in a transparent container from which the air was removed. However, this presented another challenge – the uneven heating of the container walls, which caused further convection phenomena, as creating a high vacuum at the technological level of that time was extremely difficult.
These side effects had a significant impact on the outcomes of the experiments due to the minuscule force of light pressure. This point was duly noted by scientific adversaries.
Lebedev successfully addressed all the challenges, albeit after four years of arduous effort. Initially, he focused on improving the efficiency of air removal from the vessel, which was quite innovative at that time. To minimize convection, he opted for vessels with larger capacities, effectively mitigating the impact of air movement. He also employed light filters to regulate the heating of the vessel by selectively filtering out a portion of the light spectrum that is highly absorbed by the glass walls. Additionally, he managed to overcome other factors that could potentially affect the measurement accuracy.
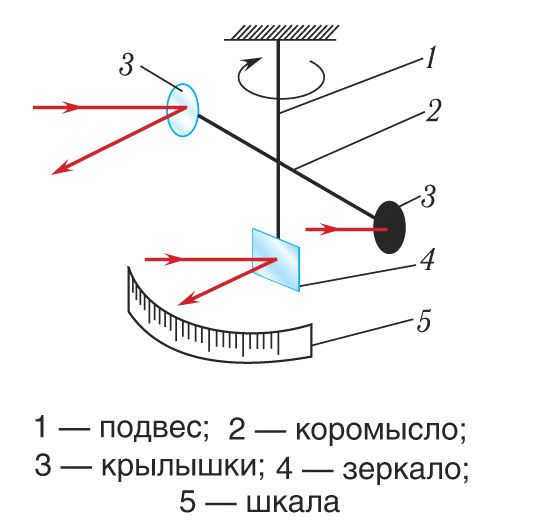

Lebedev conducted an experiment in which he exposed platinum foil wings of various thicknesses to visible light. Some of the surfaces were polished to a reflective state, while others were blackened. This experiment aimed to investigate the hypothesis that light exerts more pressure during reflection than during absorption.
The “spit” wings were suspended within a vessel using threads, and the angle of twist in these threads was used to determine the strength of light pressure. A mirror with a scale was employed to measure this angle.
During that period, the electromagnetic theory of light held sway, and the explanation for pressure was provided from the perspective of Maxwell. According to this theory, the force of pressure is directly proportional to the energy of the light beam. In order to quantify this energy, the physicist employed a calorimeter, which was temporarily substituted for the fidget spinner. Lebedev’s experiments validated the accuracy of Maxwell’s views, and the results of the experiments aligned with the calculated data to within a 20% margin of error. This was a significant breakthrough at the time.
A few months later, the Russian scientist enhanced the experimental setup, achieving a deviation from the theory of less than 1%.
Following a brief period, the theory and corpuscular (as well as electromagnetic) nature of light was widely accepted and verified in the field of physics. Lebedev’s experiments were in alignment with this theory and, to a large extent, provided further substantiation.
Summary
The concept of light pressure as a physical phenomenon has been widely accepted and acknowledged. In precise calculations of satellite paths, the impact of sunlight pressure is taken into consideration.
However, the practical utilization of this phenomenon is currently limited. While the idea of space travel propelled by the pressure of sunlight remains a distant dream, there has been a successful launch of an experimental research object to Venus that operates on a similar principle. The advancement of propulsion systems like these will require the development of robust and lightweight materials.

In nuclear physics, this phenomenon is also utilized to propel small objects to speeds below that of light. The invention of “light tweezers” for microscopic objects, although promising, is not widely applicable. However, it’s worth noting that electricity, television, and many other aspects that have become integral parts of modern life were initially seen as useless toys. Therefore, it’s necessary to wait for the advancement of technology to fully understand its potential.

Pyotr Nikolayevich Lebedev (March 8, 1866 – March 1, 1912) is widely recognized as the first renowned physicist from Russia. Lebedev is most renowned for his influential work in the experimental examination of waves. He achieved a significant milestone by successfully measuring the pressure exerted by light on a solid in 1900, thus providing empirical evidence for Maxwell’s theory. In addition to his scientific achievements, Lebedev was highly committed to popularizing science and mentoring the next generation of Russian scientists. Tragically, his untimely demise may have prevented him from being awarded the Nobel Prize, for which he had been nominated. A man of strong principles, he even resigned from his position at Moscow University when Tsar Nicholas II attempted to impose restrictions on the university’s academic freedom.
Life Story
Lebedev was born in 1866 into a family of merchants. In 1884, he enrolled in the Moscow Higher Engineering School. Although he wasn’t interested in pursuing a career as a civil engineer, the technical experience he gained from his studies proved to be valuable for his own experiments. In 1887, he joined the University of Strasbourg, which was renowned for its physics program at the time. His mentor at Strasbourg was August Kundt (1839-1894), the inventor of a method for determining the velocity of gases and solids. However, when Kundt moved to the University of Berlin in 1888, Lebedev couldn’t follow him as he didn’t have the necessary educational certificate (equivalent to a high school diploma at the time). Instead, he continued his research under the guidance of W. Kohlrausch, a physicist known for his work on the practical applications of electricity. Following Kohlrausch’s suggestion, Lebedev wrote a paper on the dielectric permittivity of vapors in 1891, which earned him a doctoral degree.
Career
Upon completing his doctorate, Lebedev relocated to Moscow and commenced his career as a laboratory assistant at Moscow University’s physical laboratory, where he worked under the guidance of A.G. Stoletov. Despite the limited resources available to him, Lebedev conducted extensive research on the resonance effect induced by electromagnetic, hydrodynamic, and acoustic waves. As a result of his groundbreaking studies, he was conferred with a Doctorate in Physical and Mathematical Sciences in 1999 and subsequently appointed as a professor at Moscow University in 1900.
Lebedev’s work on the pressure exerted by light gained worldwide recognition as evidence of the electromagnetic nature of light, thus supporting the theories of James Clerk Maxwell (1831-1879). One of the first practical applications of this phenomenon was Lebedev’s explanation of the behavior of comets in relation to the Sun’s gravity and solar wind. This idea also sparked the concept of solar sail spaceships in the science fiction community, which is now no longer purely fictional.
Around the same time, Lebedev also started investigating the Earth’s magnetism.
In 1911, Lebedev, along with several other professors, left Moscow University as part of a campaign against tsarist policies that aimed to suppress university autonomy. He continued his research in a private laboratory with his students.
In 1912, Lebedev was nominated for the Nobel Prize, competing against Einstein. According to reports from that time, Lebedev had a stronger chance of winning because his research was backed by solid empirical evidence. Tragically, on March 1, 1912, he passed away following a heart attack.
His Impact
Aside from his direct scientific accomplishments, he is remembered in Russia for his efforts in popularizing the field of physical science through his engaging lectures and informative articles. He is also recognized for his role in educating and inspiring the next generation of Russian physicists. During the year 1905, his laboratory housed approximately 20 young scientists, an impressive number considering the prevalent illiteracy that plagued Russia at the time. Furthermore, he established a lasting tradition of fostering strong connections between pure scientific research and its practical applications, often referred to as the “Lebedev school”. His unwavering determination to continue his research privately, even in the face of potential restrictions on academic freedom imposed by the state, demonstrates his moral courage and commitment to his work. The Lebedev Physical Institute bears his name, serving as a testament to his lasting impact. The P.N. Lebedev Physics Institute is also named in his honor.
literature utilized
- Dukov, V. Pyotr Nikolaevich Lebedev (Individuals of Russian science). translated by D. Skvirsky, Moscow: Foreign Languages. Dom, 1956. ASIN B0006D8E86; Honolulu, Hawaii: University Press of the Pacific, 2004. ISBN 1410216888
- Gribbin, John. Q IS FOR QUANTUM: an encyclopedia of elementary particle physics. New York: Free Press, 2000. ISBN 0684863154
- Lebedev, Pyotny N. Experimental investigation of the pressure of light. Washington, D.C., 1903.
All links retrieved on June 16, 2019.
- “Pyotr Nikolayevich Lebedev” Information sourced from Farlex.
- “Pyotr Nikolayevich Lebedev” Information sourced from the online version of Encyclopedia Britannica.
Electromagnetic radiation pressure, also known as light pressure, refers to the force exerted by light (and other forms of electromagnetic radiation) on the surface of an object.
Historical Background
The concept of light pressure was initially proposed by I. Kepler in the 17th century to explain the behavior of comet tails as they pass close to the Sun. In 1873, Maxwell developed a theory of light pressure as part of his classical electrodynamics. The first experimental investigation of light pressure was conducted by P. N. Lebedev in 1899. In his experiments, Lebedev suspended rotary scales on a thin silver thread inside a vacuum vessel. Thin disks made of mica and various metals were attached to the scales. The main challenge was distinguishing the effect of light pressure from radiometric and convective forces (forces caused by the temperature difference between the illuminated and unilluminated sides of the scales). By irradiating different sides of the disks alternately, Lebedev minimized the impact of radiometric forces and achieved a satisfactory agreement (±20%) with Maxwell’s theory. Later, between 1907 and 1910, Lebedev conducted more precise experiments on light pressure in gases and obtained further agreement with the theory [1].
Physical significance
According to modern concepts, light exhibits corpuscular-wave duality, meaning it displays both particle-like properties (photons) and wave-like properties (electromagnetic radiation).
If we consider light as a stream of photons, then, according to the principles of classical mechanics, when these particles collide with an object, they transfer momentum to it, resulting in a force known as radiation pressure.
To calculate the pressure exerted by light, we can use the following formula:
where – the amount of radiant energy incident perpendicularly on 1 m² of surface in 1 s; – the speed of light, – the reflection coefficient.
If light strikes the surface at an angle to the normal, the pressure can be expressed using the following formula:
The radiation’s volumetric energy density is represented by \(
ho\), the reflection coefficient is denoted by \(R\), the incident beam’s unit vector is \(i\), and the reflected beam’s unit vector is \(r\).
For instance, the tangential component of the force exerted by light pressure on a unit area can be calculated as:

Applications
Possible applications include the utilization of solar sails and the separation of gases [1].
Notes
Light is an incredibly valuable resource. Thanks to light, we are able to perceive the world around us, streetlights illuminate our paths, and soap bubbles display beautiful patterns. However, the composition of light remains an intriguing inquiry.

Corpuscular-wave duality.
The question that nobody will give you a definite answer to is: “Is light a particle or a wave?”. This is a very complex question that scientists have been attempting to answer for a considerable amount of time.
In the 17th century, Isaac Newton proposed a theory in which light is composed of tiny corpuscles (particles). This theory provided a simple explanation for many of the characteristic properties of light. For instance, the straightness of light rays and the law of reflection, which states that the angle of reflection is equal to the angle of incidence. This is consistent with the law of conservation of momentum, which particles adhere to.
However, there are also phenomena such as interference and diffraction that do not align with the corpuscular theory.
Caution: there are many more intricate concepts! Nevertheless, in the 10th grade elective physics course, you can grasp even the most intricate material with the guidance of a knowledgeable instructor.
Interference and diffraction
Interference occurs when two waves overlap and create regions of light and dark called “maxima” and “minima”. Here is an illustration:
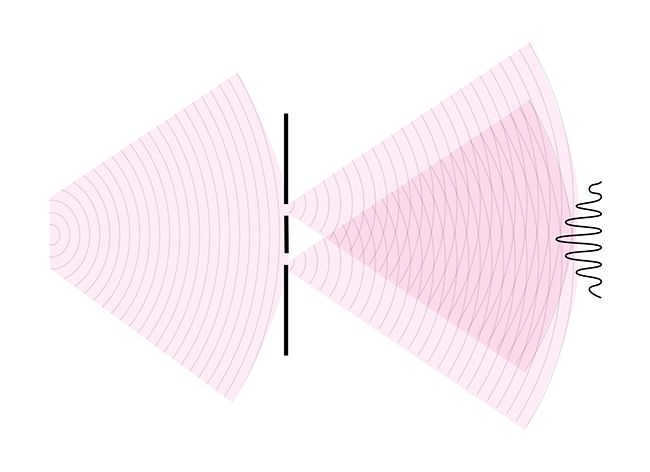
You may have come across this phenomenon in everyday life, such as when observing spilled gasoline or blowing soap bubbles. These occurrences are all a result of the interference of light.
Diffraction is closely related to the phenomenon of interference. In fact, diffraction itself is often regarded as a form of wave interference that occurs when waves are confined in a particular space.
Diffraction is the phenomenon by which waves are able to bend around obstacles that they encounter. Thanks to diffraction, light is able to bypass obstacles and reach areas where, based on geometric principles, a shadow should be formed.
At that particular time, wave optics was regarded as a miraculous concept since it not only provided explanations for phenomena that the corpuscular theory failed to explain, but also accounted for all the known light effects of that era. Furthermore, wave optics was even capable of proving the laws of geometrical optics.
One might assume that this settled the matter and concluded that light solely possessed wave-like characteristics, without any particle nature. However, this was not the case! In the early 20th century, the corpuscular theory of light once again gained significance as scientists encountered phenomena that could not be explained by the wave theory. For instance, the pressure of light and the photoelectric effect, which we will delve into later.
Within the corpuscular theory, these phenomena were effectively explained, and the particles of light were even given the name – photons.
There was a fascinating scenario – two significant scientific theories coexisted simultaneously, each providing explanations for certain properties of light, yet unable to account for others. When combined, these two theories complement each other flawlessly. Thus, the notion of the corpuscular-wave nature of light emerged.
Corpuscular-wave duality is a fundamental principle in physics that asserts that any natural object can exhibit characteristics of both a particle and a wave.


The energy and momentum of a photon
Each photon carries a certain amount of energy. This amount is referred to as the photon’s energy.
The energy of a photon (Planck-Einstein relation)
The momentum of a photon is connected to the energy through the following relationship:
Ratio of photon momentum to photon energy
Substitute the photon energy formula instead of the photon energy formula:
We decrease the speed of light and obtain the momentum formula.
Photon momentum

Light’s Pressure
The Lorentz force is the force experienced by a particle when it moves through a magnetic field.
If we consider light as a collection of photons, we can posit that light is capable of exerting pressure. This was theorized by James Maxwell in 1873 and his hypothesis has proven to be correct.
Let’s imagine photons falling perpendicularly onto the surface of a completely black body every second. Each photon possesses momentum.
The total momentum acquired by the surface of the body is equal to .
According to principles in mechanics, pressure is defined as the ratio of force to the area upon which the force acts: .
Do not be confused: impulse and pressure are represented by the same symbol, but they are distinct quantities!
Newton’s second law can be expressed in the form of an impulse equation, where I represents the impulse and t represents the time interval during which the impulse changes to the value of p.
The definition of light pressure is given by the equation: P = F/A, where P is the pressure, F is the force, and A is the area.
When light falls on a mirror surface, the impact of the photon is considered to be perfectly elastic, resulting in a change in momentum and pressure that is twice as large as when it falls on a black surface (where the impact is inelastic due to the absorption of the photon).
The existence of light pressure, as predicted by Maxwell, was confirmed experimentally by physicist P.N. Lebedev in 1900. Using sensitive torsion scales, Lebedev measured the pressure of light on solid bodies, and the results matched the theoretical predictions. The value of light pressure was approximately 4-10^-6 Pa.
Lebedev’s experiments provide experimental evidence for the fact that photons possess momentum.
Photoeffect
The photoeffect is another significant occurrence that provides evidence for the particle-like behavior of light. At this moment, we will solely focus on understanding the underlying principle of this phenomenon, while reserving the intricate mathematics for future exploration. 😉
Illustrated below is an experimental arrangement designed to investigate the photoeffect.
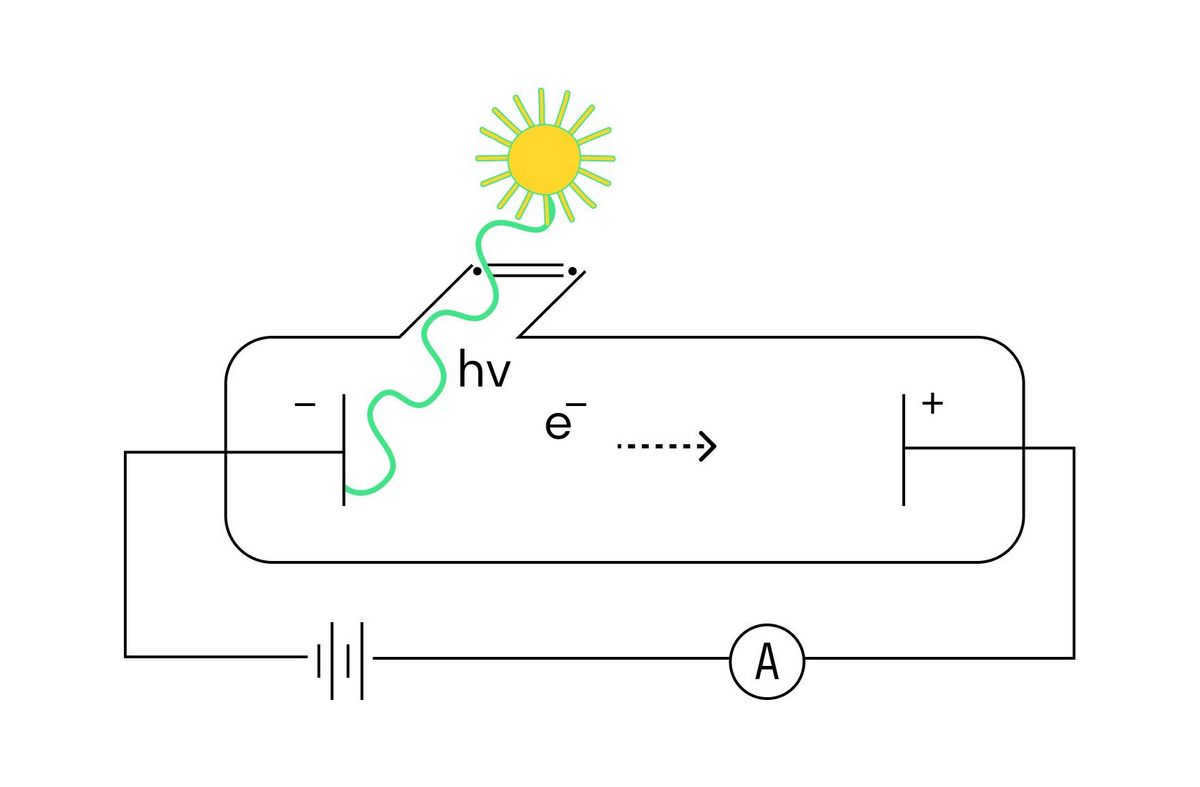
The configuration involves a glass vacuum cylinder equipped with a pair of metal electrodes that are subjected to a voltage. One of the electrodes, accessed through a quartz window, is illuminated with monochromatic light, which refers to light with a consistent wavelength. When photons interact with the electrode, they dislodge photoelectrons from the negatively charged electrode. These photoelectrons are then attracted towards the positive electrode, resulting in the formation of a photocurrent.
Various researchers have discovered the fundamental patterns of the photoelectric effect:
- The maximum kinetic energy of the photoelectrons increases proportionally with the frequency of the light, regardless of its intensity.
- Each substance exhibits a red limit for the photoelectric effect, indicating the minimum frequency at which the external photoeffect is still viable.
- The intensity of light directly determines the number of photoelectrons emitted by the cathode within a span of 1 second.
- When the cathode is illuminated, the photoelectric effect occurs instantaneously without any delay, as long as the frequency of light is maintained.
Einstein extensively studied the photoelectric effect and concluded that light is composed of photons, which indicates its discontinuous nature.
The photoelectric effect finds practical application in light sensors, such as those used in street lights that automatically switch on when a specific level of natural light is reached.
Practical Applications of Photons in Technology
The laser is a highly significant technological tool that relies on the use of photons. It finds extensive applications in various fields, including metal cutting, cooking, and melting, as well as the production of ultrapure metals. Additionally, lasers serve as the foundation for precise physical instruments like seismographs. Laser printers and pointers are also quite common in everyday life.
Photon-based random number generators are widely employed as well. These generators operate by sending a photon to a beam splitter, which separates light into two streams. In this process, there are only two possible outcomes for each photon, with an equal chance of either passing through the beamsplitter or being reflected off its edge. Depending on whether the photon passes through or not, a 0 or 1 is written as the next bit in the generated random sequence.





